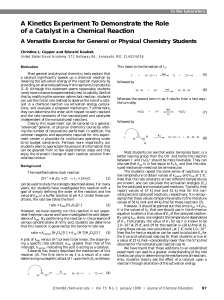
Thermodynamics
... Defines systems using a few macroscopic (measurable) variables, such as internal energy, entropy, temperature and pressure Statistical treatment of microstates (atom positions and velocities) to obtain macrostates In chemistry, thermodynamics predicts if reactions occur, how the equilibrium co ...
... Defines systems using a few macroscopic (measurable) variables, such as internal energy, entropy, temperature and pressure Statistical treatment of microstates (atom positions and velocities) to obtain macrostates In chemistry, thermodynamics predicts if reactions occur, how the equilibrium co ...
Chemical Reactions PPT
... type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction ...
... type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction ...
200 Ways to Pass the Chemistry
... 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron leaving 2 below it 98. Covalent ...
... 97. Atoms are most stable when they have 8 valence electrons (an octet) and tend to form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl It loses its 1 valence electron leaving 2 below it 98. Covalent ...
Industriel katalys
... John F. Hartwig, Organotransition Metal Chemistry – From Bonding to Catalysis; ...
... John F. Hartwig, Organotransition Metal Chemistry – From Bonding to Catalysis; ...
Thermochemistry
... and chemical changes occur because that is the path of least resistance. The production and usage of energy that occurs in reactions have enormous impacts on society. People are making big bucks as they try to find new ways to harness energy (think of nuclear energy, solar energy, and fossil fuels). ...
... and chemical changes occur because that is the path of least resistance. The production and usage of energy that occurs in reactions have enormous impacts on society. People are making big bucks as they try to find new ways to harness energy (think of nuclear energy, solar energy, and fossil fuels). ...
200 Things to Know to Pass the Chemistry Regents
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
200 Ways to Pass the Chemistry
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
200things2know
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
... form ions to obtain such a configuration of electrons. Which of the following atoms forms a stable ion that does not have an octet structure? Li F Na Cl 98. Covalent bonds form when two atoms share a pair of electrons. How many covalent bonds are found in a nitrogen (N2) molecule? 99. Ionic bonds fo ...
Physical and Chemical Changes
... the total amount of energy stays the same. The word conservation comes from conserve which means “to protect from loss.” Energy can change from one form to another but can never be lost. For example, an unlit match contains chemical energy. When it is lit, the chemical energy changes to light and th ...
... the total amount of energy stays the same. The word conservation comes from conserve which means “to protect from loss.” Energy can change from one form to another but can never be lost. For example, an unlit match contains chemical energy. When it is lit, the chemical energy changes to light and th ...
Lecture 5: Spectroscopy and Photochemistry I
... – Absorption of light can occur if dipole moment changes during vibration. E.g. HCl, CO, NO – Homonuclear diatomics, e.g. O2, N2 don’t have v.t. ...
... – Absorption of light can occur if dipole moment changes during vibration. E.g. HCl, CO, NO – Homonuclear diatomics, e.g. O2, N2 don’t have v.t. ...
H Why - Yale University
... The values of bond dissociation energies and average bond energies, when corrected for certain “effects” (i.e. predictable errors) can lead to understanding equilibrium and rate processes through statistical mechanics. The Boltzmann factor favors minimal energy in order to provide the largest number ...
... The values of bond dissociation energies and average bond energies, when corrected for certain “effects” (i.e. predictable errors) can lead to understanding equilibrium and rate processes through statistical mechanics. The Boltzmann factor favors minimal energy in order to provide the largest number ...
SAMPL E PA GES
... radiation and do not allow any UV radiation through to the skin. Sun-screen products can also be used, but are less effective. They contain chemicals, such as p-aminobenzoic acid, shown opposite which absorbs some of the UV radiation so less reaches the skin. ...
... radiation and do not allow any UV radiation through to the skin. Sun-screen products can also be used, but are less effective. They contain chemicals, such as p-aminobenzoic acid, shown opposite which absorbs some of the UV radiation so less reaches the skin. ...
honors chem 6 day review packet
... contains 3 electrons in its sixth and outer main energy level the element that has 2 electrons in the p sublevel in its second main energy level 4s24p5 Be able to locate s, p, d, and f blocks on the periodic table The ___________ ____________ _____________ is the same as the period number. There are ...
... contains 3 electrons in its sixth and outer main energy level the element that has 2 electrons in the p sublevel in its second main energy level 4s24p5 Be able to locate s, p, d, and f blocks on the periodic table The ___________ ____________ _____________ is the same as the period number. There are ...
Document
... During phase changes temperature and pressure are constant. (Heat transfer is reversible, so H = q = qrev) Calculate the entropy change when 1 mole of liquid water evaporates at 100oC (Hvap = +44 kJ/mol) ...
... During phase changes temperature and pressure are constant. (Heat transfer is reversible, so H = q = qrev) Calculate the entropy change when 1 mole of liquid water evaporates at 100oC (Hvap = +44 kJ/mol) ...