Chapter 6A Chemical Reactions CHAPTER OUTLINE
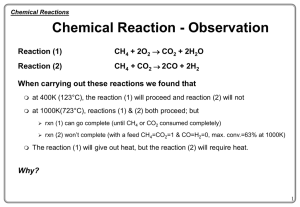
... q A chemical reaction is a rearrangement of atoms in which some of the original bonds are broken and new bonds are formed to give different chemical structures. q In a chemical reaction, atoms are neither created, nor destroyed. q A chemical reaction, as described above, is supported by Dalton ...
... q A chemical reaction is a rearrangement of atoms in which some of the original bonds are broken and new bonds are formed to give different chemical structures. q In a chemical reaction, atoms are neither created, nor destroyed. q A chemical reaction, as described above, is supported by Dalton ...
Topic2890 Thermodynamics and Kinetics A given system at
... Equation (f) is an interesting and important description of the kinetics of chemical reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, d ...
... Equation (f) is an interesting and important description of the kinetics of chemical reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, d ...
Chem163_Electrochem
... Chemical reactions involving the transfer of electrons are called oxidiation-reduction reactions or redox reactions. The broad field dedicated to the study of these reactions is known as electrochemistry. A redox reaction always consists of an oxidation reaction and a reduction reaction, hence the n ...
... Chemical reactions involving the transfer of electrons are called oxidiation-reduction reactions or redox reactions. The broad field dedicated to the study of these reactions is known as electrochemistry. A redox reaction always consists of an oxidation reaction and a reduction reaction, hence the n ...
Slide 1
... ◦ More specifically, the first law states that the changes in internal energy is equal to the difference between the energy supplied to the system as heat and the energy removed from the system as work performed on the surroundings. ...
... ◦ More specifically, the first law states that the changes in internal energy is equal to the difference between the energy supplied to the system as heat and the energy removed from the system as work performed on the surroundings. ...
EXP-7
... The Cannizzaro reaction is that of aldehydes that do not contain alpha hydrogens to give carboxylic acids and alcohols (alpha hydrogens cause an Aldol reaction to take place). This occurs in the presence of a strong base. Benzaldehyde, which does not contain alpha hydrogens, was used for this reacti ...
... The Cannizzaro reaction is that of aldehydes that do not contain alpha hydrogens to give carboxylic acids and alcohols (alpha hydrogens cause an Aldol reaction to take place). This occurs in the presence of a strong base. Benzaldehyde, which does not contain alpha hydrogens, was used for this reacti ...
Chemistry 1. The Periodic Table displays the
... atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2 and many large biological molecules are covalent. c. salt crystals such as NaCl ...
... atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2 and many large biological molecules are covalent. c. salt crystals such as NaCl ...
Activation parameters for ET
... Due to entropy mixing, the slope of the pH-dependence should be negative. Observed (total) activation entropy change The slope of the pH-dependence of the observed (total) entropy change is positive (or in some cases slightly negative) Our data indicate that the magnitude of ΔGET is at least as larg ...
... Due to entropy mixing, the slope of the pH-dependence should be negative. Observed (total) activation entropy change The slope of the pH-dependence of the observed (total) entropy change is positive (or in some cases slightly negative) Our data indicate that the magnitude of ΔGET is at least as larg ...
Exam 2 - Wake Forest University
... Do not open or begin this exam until instructed. This exam consists of 5 pages plus the cover page. Before starting the exam, check to make sure that you have all of the pages. The exam has a total of 100 points and includes 13 questions. Only legible answers written on the exam will be considered f ...
... Do not open or begin this exam until instructed. This exam consists of 5 pages plus the cover page. Before starting the exam, check to make sure that you have all of the pages. The exam has a total of 100 points and includes 13 questions. Only legible answers written on the exam will be considered f ...
Conducting Polymer
... the valence band (p-doping) or added to the conduction band (n-doping, which is far less common) does a conducting polymer become highly conductive. • Doping (p or n) generates charge carriers which move in an electric field. Positive charges (holes) and negative charges (electrons) move to opposite ...
... the valence band (p-doping) or added to the conduction band (n-doping, which is far less common) does a conducting polymer become highly conductive. • Doping (p or n) generates charge carriers which move in an electric field. Positive charges (holes) and negative charges (electrons) move to opposite ...
Review Material
... Ionic Bonding & Ions An ionic bond is formed when one electron, or more, is/are transferred from one atom to another. Positive ions are referred to as cations; negative ions are referred to as anions. ...
... Ionic Bonding & Ions An ionic bond is formed when one electron, or more, is/are transferred from one atom to another. Positive ions are referred to as cations; negative ions are referred to as anions. ...