g) Chemistry 30 - Mr. Jones LHS Science
... c. Draw a reaction coordinate diagram showing if the relative energy of the reactants and products. . Label the ∆H on the diagram. ...
... c. Draw a reaction coordinate diagram showing if the relative energy of the reactants and products. . Label the ∆H on the diagram. ...
Hydrogen Bonding
... Ionic Bonding – Electrons are transferred from one atom to another producing a charge on both atoms (ions), in order to keep them together. NaCl Covalent Bonding – Sharing of electrons between atoms resulting in an overlap of their electron orbitals. Diamond Metallic Bonding – Formed when closel ...
... Ionic Bonding – Electrons are transferred from one atom to another producing a charge on both atoms (ions), in order to keep them together. NaCl Covalent Bonding – Sharing of electrons between atoms resulting in an overlap of their electron orbitals. Diamond Metallic Bonding – Formed when closel ...
Unit 1
... 1. To know that chemical bonds, the forces that hold atoms together (text definition), are the lowering of energy when atoms come together (additional definition). 2. To describe, differentiate, and give examples of ionic, covalent, and metallic bonds. 3. To use Lewis dot symbols for atoms and ions ...
... 1. To know that chemical bonds, the forces that hold atoms together (text definition), are the lowering of energy when atoms come together (additional definition). 2. To describe, differentiate, and give examples of ionic, covalent, and metallic bonds. 3. To use Lewis dot symbols for atoms and ions ...
Chemical Reactions - Johnston County Schools
... Substances other than hydrocarbons can also combust. However, you may not be able to tell whether it’s combustion from the chemical equation alone. Remember that combustion must have O2 as a reactant and must release (exothermic) heat and light energy. Reactions with O2.mov ...
... Substances other than hydrocarbons can also combust. However, you may not be able to tell whether it’s combustion from the chemical equation alone. Remember that combustion must have O2 as a reactant and must release (exothermic) heat and light energy. Reactions with O2.mov ...
Journal of Physical and Chemical Reference Data
... number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These reactions and their associated heats of reaction can then be used to ...
... number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These reactions and their associated heats of reaction can then be used to ...
Template for calculating the ΔH° in a multiple step chemical reaction
... b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction The chemical reaction: Zn + H2SO4 → ZnSO4 + H2 is a a. synthesis reaction b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction ...
... b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction The chemical reaction: Zn + H2SO4 → ZnSO4 + H2 is a a. synthesis reaction b. decomposition reaction c. single displacement reaction d. double displacement reaction e. combustion reaction ...
Refined structure of c-phycocyanin from the cyanobacterium
... the Fremyella diplosiphon (Fd-PC) structure shows solvent molecules in similar positions, indicating that this is not characteristic of PC from thermophiles only. The positions of the propionic acids have not been indicated as important for energy transfer per se; however, by obtaining their proper ...
... the Fremyella diplosiphon (Fd-PC) structure shows solvent molecules in similar positions, indicating that this is not characteristic of PC from thermophiles only. The positions of the propionic acids have not been indicated as important for energy transfer per se; however, by obtaining their proper ...
Step 1
... Therefore more electrons are pushed onto the N atom (because of the positive inductive effect of alkyl groups). One might expect using the same trend that tertiary amine would be the strongest amine base but the trend does not hold. The tertiary amines and corresponding ammonium salts are less ...
... Therefore more electrons are pushed onto the N atom (because of the positive inductive effect of alkyl groups). One might expect using the same trend that tertiary amine would be the strongest amine base but the trend does not hold. The tertiary amines and corresponding ammonium salts are less ...
Knox Chem Prelim 2009
... Heating water to boil it simply separates its molecules, BUT electrolysis separates its atoms to form new substances. Which statement best explains this difference? (A) ...
... Heating water to boil it simply separates its molecules, BUT electrolysis separates its atoms to form new substances. Which statement best explains this difference? (A) ...
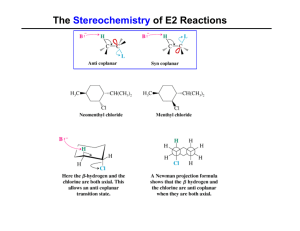
The Stereochemistry of E2 Reactions
... Hammond-Leffler Postulate: the transition state for a step that is downhill in energy should show a strong resemblance to the reactant of that step. ...
... Hammond-Leffler Postulate: the transition state for a step that is downhill in energy should show a strong resemblance to the reactant of that step. ...