Chapter 15 PPT
... Thermodynamics is the study of the changes in energy and transfers of energy that accompany chemical and physical processes. In this chapter we will address 3 fundamental questions. Will two (or more) substances react when they are mixed under specified conditions? If they do react, what energy chan ...
... Thermodynamics is the study of the changes in energy and transfers of energy that accompany chemical and physical processes. In this chapter we will address 3 fundamental questions. Will two (or more) substances react when they are mixed under specified conditions? If they do react, what energy chan ...
g - Valencia College
... a negative sign (loss of energy). (-DE or - DH) • Energy flow from the surroundings to the system has ...
... a negative sign (loss of energy). (-DE or - DH) • Energy flow from the surroundings to the system has ...
Chapter 1: Fundamental Concepts
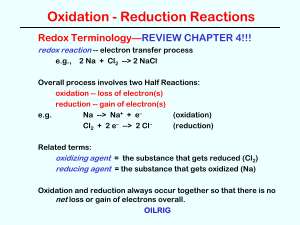
... one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Remember that if something is oxidized, something else must b ...
... one species is oxidized and one is reduced. The two processes will always occur together. • When has a redox reaction occurred? – If there is a change in the oxidation state of any element in the reaction, a redox reaction has happened. – Remember that if something is oxidized, something else must b ...
Chem1101 – Semester 1
... Recognise the consequences of Hund’s rule on the detailed electronic configuration of an atom Having one electron in each p-‐orbital will keep the electrons as far from each other as possible to accoun ...
... Recognise the consequences of Hund’s rule on the detailed electronic configuration of an atom Having one electron in each p-‐orbital will keep the electrons as far from each other as possible to accoun ...
Equilibrium
... The activation energy barrier for a catalyzed reaction is lower than that of a uncatalyzed reaction. With a lower activation energy barrier, more reactants have the energy to form products within a given time. Because a catalyst is not consumed during a reaction, it does not appear as a reactant or ...
... The activation energy barrier for a catalyzed reaction is lower than that of a uncatalyzed reaction. With a lower activation energy barrier, more reactants have the energy to form products within a given time. Because a catalyst is not consumed during a reaction, it does not appear as a reactant or ...
Chapter 4: Solution Chemistry and the Hydrosphere
... To balance and complete the precipitation reactions: 1. Exchange the anions, writing the formulas for the products based on the charges of the ions! 2. Use the Solubility Rules to determine if each product is soluble or insoluble. – If at least one product is insoluble, a precipitation reaction has ...
... To balance and complete the precipitation reactions: 1. Exchange the anions, writing the formulas for the products based on the charges of the ions! 2. Use the Solubility Rules to determine if each product is soluble or insoluble. – If at least one product is insoluble, a precipitation reaction has ...
Thermodynamics
... Direction and sign of heat flow – MEMORIZE! ENDOTHERMIC: heat is added to the system & the temperature increases (+q) EXOTHERMIC: heat is lost from the system (added to the surroundings) & the temperature in the system decreases (-q) ...
... Direction and sign of heat flow – MEMORIZE! ENDOTHERMIC: heat is added to the system & the temperature increases (+q) EXOTHERMIC: heat is lost from the system (added to the surroundings) & the temperature in the system decreases (-q) ...
File - Grade 12 Chemistry
... Other answers are possible for the alcohol and the ether. PTS: 1 9. ANS: Dispersion forces are very weak intermolecular forces that exist between molecules. When a carbon atom is bonded to another carbon atom, or to a hydrogen atom, the bond is not considered to be polar because the electronegativit ...
... Other answers are possible for the alcohol and the ether. PTS: 1 9. ANS: Dispersion forces are very weak intermolecular forces that exist between molecules. When a carbon atom is bonded to another carbon atom, or to a hydrogen atom, the bond is not considered to be polar because the electronegativit ...
Practice Exam-Final Fall 2016 W-Ans
... 16. How many hydrogen atoms are there in 48.0 g of CH4? (a) 1.81x1023 (b) 7.22x1024 (c) 6.02x1023 (d) 1.20x1025 (e) 4.70x1025 Hint: According to the chemical formula, one mole of CH4 contains 1 mole of C atoms and 4 moles of hydrogen atoms. Thus, the mole of H = 4 x {mass of CH4/molar mass of CH4}. ...
... 16. How many hydrogen atoms are there in 48.0 g of CH4? (a) 1.81x1023 (b) 7.22x1024 (c) 6.02x1023 (d) 1.20x1025 (e) 4.70x1025 Hint: According to the chemical formula, one mole of CH4 contains 1 mole of C atoms and 4 moles of hydrogen atoms. Thus, the mole of H = 4 x {mass of CH4/molar mass of CH4}. ...
Chemical Equations
... compounds by exchanging cations and anions Reactants are ionic compounds or acids, usually in aqueous solution Insoluble products will precipitate out of solution or be released as gases AKA double displacement reactions ...
... compounds by exchanging cations and anions Reactants are ionic compounds or acids, usually in aqueous solution Insoluble products will precipitate out of solution or be released as gases AKA double displacement reactions ...
Code: I1 Title: Heterogeneous Catalysis Lecturer: Prof S D Jackson
... organometallic compounds, and be able to relate these to the types of ligand in the complex. Explain synergic bonding using simple molecular orbital theory and hence explain why certain ligands such as CO are able to stabilise metals in very low oxidation states. Describe the syntheses of some super ...
... organometallic compounds, and be able to relate these to the types of ligand in the complex. Explain synergic bonding using simple molecular orbital theory and hence explain why certain ligands such as CO are able to stabilise metals in very low oxidation states. Describe the syntheses of some super ...
File
... When electrons move from one area to another they produce an electric current (electricity!). Since redox reactions involve the transfer of electrons, these chemical reactions can be used to produce electricity. ...
... When electrons move from one area to another they produce an electric current (electricity!). Since redox reactions involve the transfer of electrons, these chemical reactions can be used to produce electricity. ...
Theoretical problems (official version)
... The quantum requirement of the light redox reactions is defined as the average number of light photons (not necessarily integer) needed for the transfer of one electron from a reducing agent to an oxidant. The isolated chloroplasts were irradiated during 2 hours by a monochromatic light (wavelength ...
... The quantum requirement of the light redox reactions is defined as the average number of light photons (not necessarily integer) needed for the transfer of one electron from a reducing agent to an oxidant. The isolated chloroplasts were irradiated during 2 hours by a monochromatic light (wavelength ...