2016
... For those students who have already taken a high school chemistry course, much of the material in the summer packet will be familiar to you. For those students who will be taking AP Chemistry as your first high school chemistry course, the problems will help you build a foundation in chemistry and i ...
... For those students who have already taken a high school chemistry course, much of the material in the summer packet will be familiar to you. For those students who will be taking AP Chemistry as your first high school chemistry course, the problems will help you build a foundation in chemistry and i ...
Introduction
... Now that we know something about atoms and molecules, it's time to see what kinds of reactions they can undergo and how we can describe these reactions. This unit will cover chemical equations, the nature of solutes in aqueous solutions and a few types of common reactions. A balanced chemical equati ...
... Now that we know something about atoms and molecules, it's time to see what kinds of reactions they can undergo and how we can describe these reactions. This unit will cover chemical equations, the nature of solutes in aqueous solutions and a few types of common reactions. A balanced chemical equati ...
answers to part a of the national high school
... the symbol, or they could have calculated the molar mass from the formula (but in the latter case they would have lost precious time). The formula given in the question is MnSiO3 3Mn2O3, and the question focuses on understanding what this type of formula means. The dot in the middle of the formula ...
... the symbol, or they could have calculated the molar mass from the formula (but in the latter case they would have lost precious time). The formula given in the question is MnSiO3 3Mn2O3, and the question focuses on understanding what this type of formula means. The dot in the middle of the formula ...
Basic Background Review: Acid-Base , Redox, and Stable Isotopes
... 1. All the major bioactive elements (except P) have multiple stable isotopes. 2. Within this group, the light isotope (L) is consistently more abundant than the heavy (H) counterpart(s). 3. It is very small (ppt) differences in (H/L) that constitute the basis of using stable isotope signatur ...
... 1. All the major bioactive elements (except P) have multiple stable isotopes. 2. Within this group, the light isotope (L) is consistently more abundant than the heavy (H) counterpart(s). 3. It is very small (ppt) differences in (H/L) that constitute the basis of using stable isotope signatur ...
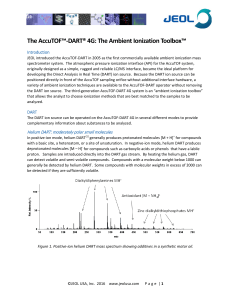
The Ambient Ionization Toolbox
... with a basic site, a heteroatom, or a site of unsaturation. In negative-ion mode, helium DART produces deprotonated molecules [M – H]- for compounds such as carboxylic acids or phenols that have a labile proton. Samples are introduced directly into the DART gas stream. By heating the helium gas, DAR ...
... with a basic site, a heteroatom, or a site of unsaturation. In negative-ion mode, helium DART produces deprotonated molecules [M – H]- for compounds such as carboxylic acids or phenols that have a labile proton. Samples are introduced directly into the DART gas stream. By heating the helium gas, DAR ...
슬라이드 1
... • Molecular hopping requires an activation energy Ea. • Consider a liquid contained between two plates, one is moving and the other is fixed. • When the plate is moving with the 1st layer, the 2nd layer molecules (eg. blue circle) feels tangential force // to v by the attractive interaction with the ...
... • Molecular hopping requires an activation energy Ea. • Consider a liquid contained between two plates, one is moving and the other is fixed. • When the plate is moving with the 1st layer, the 2nd layer molecules (eg. blue circle) feels tangential force // to v by the attractive interaction with the ...
fahad h. ahmad - Fahad`s Academy
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
... 1. Ionic compounds are hard crystalline solids with flat sides and regular shapes because the ions are arrnged in straight rows in strong ionic bonds. 2. Ionic compounds have very high melting points and boiling points. 3. The strong forces holding ionic compounds prevents them to evaporate easily. ...
Acrobat - chemmybear.com
... 5 • Reactions in Aqueous Solution STUDY QUESTIONS AND PROBLEMS 1. Classify each of the following solutes as a strong electrolyte, weak electrolyte, or nonelectrolyte: sugar sodium hydroxide common salt (NaCl) hydrochloric acid alcohol copper sulfate acetic acid carbonic acid 2. Predict the solubilit ...
... 5 • Reactions in Aqueous Solution STUDY QUESTIONS AND PROBLEMS 1. Classify each of the following solutes as a strong electrolyte, weak electrolyte, or nonelectrolyte: sugar sodium hydroxide common salt (NaCl) hydrochloric acid alcohol copper sulfate acetic acid carbonic acid 2. Predict the solubilit ...
Basic Concepts of the Gas Phase
... Ions are atoms or groups of atoms that possess a net charge. When one or more electrons are thermally or chemically removed from an atom, ions are formed. Anions, e.g. Cl-, are negatively charged. Cations, e.g. Na+, are positively charged. Coulomb forces between positive and negative charges attract ...
... Ions are atoms or groups of atoms that possess a net charge. When one or more electrons are thermally or chemically removed from an atom, ions are formed. Anions, e.g. Cl-, are negatively charged. Cations, e.g. Na+, are positively charged. Coulomb forces between positive and negative charges attract ...
che-20028 QC lecture 1 - Rob Jackson`s Website
... How the experiment is performed • Using a variable frequency light source, shine light onto a metal surface. • Determine the light frequency which causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balanc ...
... How the experiment is performed • Using a variable frequency light source, shine light onto a metal surface. • Determine the light frequency which causes electrons to be emitted. • Measure the energy of the emitted electrons, by applying a voltage across the cell in the opposite direction to balanc ...
Electron domain and molecular geometry of bro2-
... the molecular geometry of ClO3- including a description of the ClO3- bond angles. Looking at the ClO3- Lewis structure we can see. A quick explanation of the molecular geometry of SO2 including a description of the SO2 bond angles. We can see that there are only two atoms attached to. The molecular ...
... the molecular geometry of ClO3- including a description of the ClO3- bond angles. Looking at the ClO3- Lewis structure we can see. A quick explanation of the molecular geometry of SO2 including a description of the SO2 bond angles. We can see that there are only two atoms attached to. The molecular ...
Chapter 1 - TamAPChemistryHart
... 15. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solid ...
... 15. A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solid ...
Chemistry I Exams and Keys Corrected 2016 Season
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
... 18. Joseph Proust(1754 to 1826) was the chemist to first formally state that: Rejected: because simple memorization. Also, student may not have read about Proust. All full credit. A) When two elements combine with each other to form more than one compound, the weights of one element that combine wi ...
Franck-Hertz Effect in Mercury
... The next level above this is the "triplet" 3P0 level (6s6p), with the lowest member (first excited state) at 4.667 electron volts (eV). Photon de-excitation transitions from this to the ground state are not allowed, by the requirement of vector angular momentum conservation (the total angular moment ...
... The next level above this is the "triplet" 3P0 level (6s6p), with the lowest member (first excited state) at 4.667 electron volts (eV). Photon de-excitation transitions from this to the ground state are not allowed, by the requirement of vector angular momentum conservation (the total angular moment ...
23. Oxidation and Reduction
... Oxidation and reduction are processes that must occur together. This is true because it is impossible to create or destroy electrons in any chemical change. Electrons can only be transferred from one substance to another. The electrons that are lost in oxidation are gained in a reduction reaction. T ...
... Oxidation and reduction are processes that must occur together. This is true because it is impossible to create or destroy electrons in any chemical change. Electrons can only be transferred from one substance to another. The electrons that are lost in oxidation are gained in a reduction reaction. T ...
Chemistry - Ysgol Bro Pedr
... lowest energy level. The diagram to the left shows how we drew electronic structure at GCSE – electrons appeared on the shells. ...
... lowest energy level. The diagram to the left shows how we drew electronic structure at GCSE – electrons appeared on the shells. ...
chemistry
... and identical properties. (2) They have identical molecular and different properties. (3) They have different molecular and identical properties. (4) They have different molecular and different properties. ...
... and identical properties. (2) They have identical molecular and different properties. (3) They have different molecular and identical properties. (4) They have different molecular and different properties. ...
Key - GCC
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
Electron bubbles in liquid 4He containing a small
... as a constant and that the outward electrostatic force on an area can be balanced by the surface tension. Equations (1) and (2) are also based on the approximation that the electrons are confined to a layer of infinitesimal thickness just inside the wall of the bubble. Quantum zero-point motion resu ...
... as a constant and that the outward electrostatic force on an area can be balanced by the surface tension. Equations (1) and (2) are also based on the approximation that the electrons are confined to a layer of infinitesimal thickness just inside the wall of the bubble. Quantum zero-point motion resu ...
Oxidation-Reduction and Electrochemistry
... Faraday also defined a number of terms: The anode is therefore that surface at which the electric current, according to our present expression, enters: it is the negative extremity of the decomposing body; is where oxygen, chlorine, acids, etc., are evolved; and is against or opposite the positiv ...
... Faraday also defined a number of terms: The anode is therefore that surface at which the electric current, according to our present expression, enters: it is the negative extremity of the decomposing body; is where oxygen, chlorine, acids, etc., are evolved; and is against or opposite the positiv ...
Oxidation numbers
... 1. Deduce the oxidation numbers of iodine in IO3-, I– and I2. 2. The following is an equation for a redox reaction. 2NO + 12H+ + 10I– → 2NH4+ + 2H2O + 5I2 (i) Define oxidation in terms of electrons. (ii) Deduce the oxidation state of nitrogen in NO and of nitrogen in NH4+ (iii) Identify the species ...
... 1. Deduce the oxidation numbers of iodine in IO3-, I– and I2. 2. The following is an equation for a redox reaction. 2NO + 12H+ + 10I– → 2NH4+ + 2H2O + 5I2 (i) Define oxidation in terms of electrons. (ii) Deduce the oxidation state of nitrogen in NO and of nitrogen in NH4+ (iii) Identify the species ...
Topic 9 - Anderson High School
... – O = -2 (except in H2O2 where its +1) – H = +1 (except in hydrides H-) – Halides = -1 except when bonded to oxygen or other halides higher in the group (more reactive one will be -1) ...
... – O = -2 (except in H2O2 where its +1) – H = +1 (except in hydrides H-) – Halides = -1 except when bonded to oxygen or other halides higher in the group (more reactive one will be -1) ...
bioinorganic 1
... Electron transfer normally involves movement of one electron at a time (the ultimate use is usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Ir ...
... Electron transfer normally involves movement of one electron at a time (the ultimate use is usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Ir ...
10.3 Ligand Field Theory 10.3 Ligand Field Theory
... - Insertion of d orbitals w/ ligands orbitals splitting of orbitals E t2g sets: lowered E byy -2/5 ∆o eg sets: increased E by 3/5 ∆o ex) d1 system: -2/5 ∆o d4 system (high spin): 3/5 ∆o + 3(-2/5 ∆o) = -3/5 ∆o ...
... - Insertion of d orbitals w/ ligands orbitals splitting of orbitals E t2g sets: lowered E byy -2/5 ∆o eg sets: increased E by 3/5 ∆o ex) d1 system: -2/5 ∆o d4 system (high spin): 3/5 ∆o + 3(-2/5 ∆o) = -3/5 ∆o ...
Review Package KCI 2017 Sem 1
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...