First 9 weeks Study Guide 8th Grade
... A substance that consists of two or more different elements is a compound. Living matter is made up mostly of oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus which form organic compounds. Elements ...
... A substance that consists of two or more different elements is a compound. Living matter is made up mostly of oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus which form organic compounds. Elements ...
Nugget
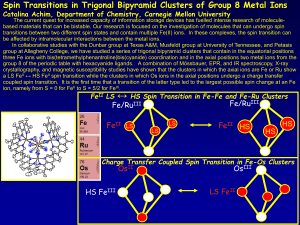
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
Solon City Schools
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
Chapter 2
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
... 1. There are no values on the y axis in the tables above. Using the Periodic Table and Table 1, put numbers on the y axis. 2. Label each peak on the graphs above with s, p, d, or f to indicate the suborbital they represent.. 3. What is the total number of electrons in a neutral potassium atom? ...
1st Semester Practice Test
... 72. What is the energy required to remove an electron from an atom in the gaseous state called? a. nuclear energy c. shielding energy b. ionization energy d. electronegative energy 73. What type of ions have names ending in -ide? a. only cations c. only metal ions b. only anions d. only ...
... 72. What is the energy required to remove an electron from an atom in the gaseous state called? a. nuclear energy c. shielding energy b. ionization energy d. electronegative energy 73. What type of ions have names ending in -ide? a. only cations c. only metal ions b. only anions d. only ...
Unit 2 Practice Exam exam_2p_08_matter
... c. atoms of an element are identical in mass. d. atoms combine in simple whole number ratios to form compounds. 7. Which of the following is NOT an update to Dalton’s atomic theory? a. Atoms are divisible and composed of smaller particles. b. Atoms can be converted into other types in a nuclear reac ...
... c. atoms of an element are identical in mass. d. atoms combine in simple whole number ratios to form compounds. 7. Which of the following is NOT an update to Dalton’s atomic theory? a. Atoms are divisible and composed of smaller particles. b. Atoms can be converted into other types in a nuclear reac ...
No Slide Title
... Group 8A Elements (ns2np6, n 2) Completely filled ns and np subshells. Highest ionization energy of all elements. No tendency to accept extra electrons. ...
... Group 8A Elements (ns2np6, n 2) Completely filled ns and np subshells. Highest ionization energy of all elements. No tendency to accept extra electrons. ...
Chapter 2 - Chemical Context of Life
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
Chemistry Test Review - Greenslime Home Page
... a. That it has 15 protons and 15 electrons. 2. What is the number of neutrons in a neutral atom of Rubidium? a. 48 3. Describe the most reactive and least reactive families of the periodic table. a. Most reactive – Hydrogen family – have 1 valence electron – all act like metals – highly reactive b. ...
... a. That it has 15 protons and 15 electrons. 2. What is the number of neutrons in a neutral atom of Rubidium? a. 48 3. Describe the most reactive and least reactive families of the periodic table. a. Most reactive – Hydrogen family – have 1 valence electron – all act like metals – highly reactive b. ...
Ionic bonding
... NaCl is a 3D array of Na+ and Cl- ions that form a repeating array, aka a crystal. • The size of the crystal depends on the number of salt units. Size can increase! How does NaCl form? Na loses an e-, and that same e- is transferred to Cl. • Both atoms become ions and are oppositely charged: Na+1 & ...
... NaCl is a 3D array of Na+ and Cl- ions that form a repeating array, aka a crystal. • The size of the crystal depends on the number of salt units. Size can increase! How does NaCl form? Na loses an e-, and that same e- is transferred to Cl. • Both atoms become ions and are oppositely charged: Na+1 & ...
File - docstover.org
... Objective 1: Students will be able to define matter, differentiate between its different states, and understand how it remains constant with a system. ...
... Objective 1: Students will be able to define matter, differentiate between its different states, and understand how it remains constant with a system. ...
THE UNIVERSITY OF LETHBRIDGE DEPARTMENT OF CHEMISTRY
... Oxide ions are at the cube corners and in the cube center. The central oxide ion is tetrahedrally surrounded by four copper ions. a) What is the formula of cuprite? Justify your answer. 8 corner O2- x 1/8th = 1 1 central O2– = 1 4 internal Cu ions. Therefore the formula is Cu2O, b) What is the oxida ...
... Oxide ions are at the cube corners and in the cube center. The central oxide ion is tetrahedrally surrounded by four copper ions. a) What is the formula of cuprite? Justify your answer. 8 corner O2- x 1/8th = 1 1 central O2– = 1 4 internal Cu ions. Therefore the formula is Cu2O, b) What is the oxida ...
Atomic Structure. Chemical Bonds.
... Atoms are held together by electric forces Three types of chemical bonds: ...
... Atoms are held together by electric forces Three types of chemical bonds: ...
Ch. 2: The Chemical Context of Life AP Reading Guide
... 1. Define and give an example of the following terms: matter, element, compound. 2. What four elements make up 96% of all living matter? 3. What is the difference between an essential element and a trace element? Concept 2.2 An element’s properties depend on the structure of its atoms 4. Sketch a mo ...
... 1. Define and give an example of the following terms: matter, element, compound. 2. What four elements make up 96% of all living matter? 3. What is the difference between an essential element and a trace element? Concept 2.2 An element’s properties depend on the structure of its atoms 4. Sketch a mo ...
Honors Chemistry
... 59. Sodium hypochlorite, the main ingredient in household bleach, is produced according the following reaction. Sodium hydroxide + chlorine → sodium chloride + sodium hypochlorite + water What is the percent yield of the reaction if 1.25 kg of chlorine reacts to form 0.90 kg of sodium hypochlorite? ...
... 59. Sodium hypochlorite, the main ingredient in household bleach, is produced according the following reaction. Sodium hydroxide + chlorine → sodium chloride + sodium hypochlorite + water What is the percent yield of the reaction if 1.25 kg of chlorine reacts to form 0.90 kg of sodium hypochlorite? ...
Chem 101 notes review
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
Outline Chapter 10 The Periodic Law
... 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound fo ...
... 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a metal atom combines with a nonmetal atom to form an ionic compound, the chemical formula of the ionic compound fo ...
California Chemistry Standards Test
... a. base b. metal c. acid d. salt Which of the following elements would combine w/ chlorine to form an ionic bond a. Ar b. S c. Si d. Mg The formula for the hydronium ion is a. H+ b. H3O+ c. OH- d. HCa5(PO4)3 is held together by a. freely moving electrons b. hydrogen bonds between molecules c. shared ...
... a. base b. metal c. acid d. salt Which of the following elements would combine w/ chlorine to form an ionic bond a. Ar b. S c. Si d. Mg The formula for the hydronium ion is a. H+ b. H3O+ c. OH- d. HCa5(PO4)3 is held together by a. freely moving electrons b. hydrogen bonds between molecules c. shared ...
Test Specs - Blue Valley Schools
... 4. Analyze the trends of the periodic table based on electronegativity, electron configurations, ionization energy, and atomic radius. 5. Predict the electron configuration for an element based on the number of electrons. 6. Predict the electron configuration for an element based on the location on ...
... 4. Analyze the trends of the periodic table based on electronegativity, electron configurations, ionization energy, and atomic radius. 5. Predict the electron configuration for an element based on the number of electrons. 6. Predict the electron configuration for an element based on the location on ...
Practice Bypass Answers
... a) Is aluminum chloride an ionic or covalent compound? How does the [ionic or covalent, depending on what you selected] bond form? It is an ionic compound. Aluminum is a metal that has three valence electrons and low attraction for electrons. In order for it to achieve stable octet it needs to lose ...
... a) Is aluminum chloride an ionic or covalent compound? How does the [ionic or covalent, depending on what you selected] bond form? It is an ionic compound. Aluminum is a metal that has three valence electrons and low attraction for electrons. In order for it to achieve stable octet it needs to lose ...
Unit 1 - Morgan Science
... are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. ...
... are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. ...
+ 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g)
... Cation: An ion with a positive charge Anion: An ion with a negative charge Covalent Bond: A bond between two non-metals where a pair of electrons are shared. Ionic Bond: A bond between a non-metal and a metal where electrons are lost or gained. Subscript: A number that represents how many atoms of a ...
... Cation: An ion with a positive charge Anion: An ion with a negative charge Covalent Bond: A bond between two non-metals where a pair of electrons are shared. Ionic Bond: A bond between a non-metal and a metal where electrons are lost or gained. Subscript: A number that represents how many atoms of a ...
Unit 3 - Chemistry
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...
... • Expand without limit to fill any space. • _______________ - describes the gaseous state of a substance that is generally a liquid or solid at room temperature (different than a gas). ...