High School Physical Science Glossary
... certain frequencies is absorbed acceleration- the change in velocity divided by time (a = Δv / Δt) accuracy- values such as density of a specific element that are accepted norms and usually published; if a measurement taken matches accepted norms or a standard value, it is said to be accurate acid/b ...
... certain frequencies is absorbed acceleration- the change in velocity divided by time (a = Δv / Δt) accuracy- values such as density of a specific element that are accepted norms and usually published; if a measurement taken matches accepted norms or a standard value, it is said to be accurate acid/b ...
2 Types of Chemical Bonds
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
Course Syllabus - Honors Chemistry
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. a. Atomic number and atomic mass. b. Identify metals, semimetals, nonmetals, halogens, alkali metals, alkaline earth ...
Scientific Method - Virtual Medical Academy
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
... Chemistry:-The study of matter and the changes it can undergo MATTER:-Matter is any thing occupies space and has a mass. Matter------> has mass , mass to weight , occupies space. There are things you can see.. "e.x: water, tree, food". And there are things you can't see.. " air, gas in gas cylinders ...
Chemistry Midterm Review 2006
... 7. What are flame tests? What area of the electromagnetic radiation spectrum allows us to observe flame tests? Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 8. What is the difference between a ground state and an excited state? 9. What is t ...
... 7. What are flame tests? What area of the electromagnetic radiation spectrum allows us to observe flame tests? Is energy released or absorbed when an electron falls from a higher energy level to a lower energy level? 8. What is the difference between a ground state and an excited state? 9. What is t ...
PIB and HH - Unit 4 - Chemical Names and Formulas
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
... Bonded atoms attain the stable electron configuration of a noble gas. The noble gases themselves exist as isolated atoms because that is their most stable condition. For the representative elements, the number of valence electrons is equal to the element’s group number in the periodic table. The tra ...
Activity 17 Follow-up
... Activity 17 Analysis 3. Was it possible for an atom to make more than one bond? Explain, and give an ...
... Activity 17 Analysis 3. Was it possible for an atom to make more than one bond? Explain, and give an ...
Science 9
... in a 100-g beaker, a student added 25 g of lead (II) nitrate to 15 g of sodium iodide. In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
... in a 100-g beaker, a student added 25 g of lead (II) nitrate to 15 g of sodium iodide. In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
Integrated Science 3
... 19. What is true about the element immediately below the element that has an atomic number 17 in the periodic table. a) 17 electrons in its outer most level c) 17 protons in nucleus b) 7 electrons in its outermost level d) 7 protons in its nucleus 20. Two atoms that are isotopes have the same number ...
... 19. What is true about the element immediately below the element that has an atomic number 17 in the periodic table. a) 17 electrons in its outer most level c) 17 protons in nucleus b) 7 electrons in its outermost level d) 7 protons in its nucleus 20. Two atoms that are isotopes have the same number ...
lewis dot diagrams (structures) for atoms and ions predicting
... 2. Chemical bonding is the process of atoms combining to form new __________________________. 3. Matter tends to exist in its ______________________________ energy state. 4. A(n) __________________________ bond is a bond in which one atom donates electrons to another atom. 5. When the number of prot ...
... 2. Chemical bonding is the process of atoms combining to form new __________________________. 3. Matter tends to exist in its ______________________________ energy state. 4. A(n) __________________________ bond is a bond in which one atom donates electrons to another atom. 5. When the number of prot ...
General CHemistry Unit 2 Homework Notes
... The only way to form a compound from elements is by a chemical reaction. Example: Hydrogen gas and oxygen gas react to synthesize water. 2H2 + O2 2H2O The only way to separate a compound into its elements is by a chemical reaction that breaks the chemical bonds, forming new substances. (Example: w ...
... The only way to form a compound from elements is by a chemical reaction. Example: Hydrogen gas and oxygen gas react to synthesize water. 2H2 + O2 2H2O The only way to separate a compound into its elements is by a chemical reaction that breaks the chemical bonds, forming new substances. (Example: w ...
Chemistry -- Oxidation
... 6. The oxidation number of oxygen (O) is -2 in most compounds. Exceptions are O2 (where O = 0) and peroxides, such as H2O2 or Na2O2, where O = -1. • For other elements, you can usually use If no other rules apply, assume ON is the same as the charge taken on in an ionic compound (“the charge it woul ...
... 6. The oxidation number of oxygen (O) is -2 in most compounds. Exceptions are O2 (where O = 0) and peroxides, such as H2O2 or Na2O2, where O = -1. • For other elements, you can usually use If no other rules apply, assume ON is the same as the charge taken on in an ionic compound (“the charge it woul ...
Exercises 2
... Which of the following conclusions can be drawn based on the outcome of Rutherford’s experiment (scattering of a-particles by gold foil)?* a) Electrons are rotating in circles b) Electrons are not moving at all c) The volume of the nucleus is much smaller than the volume of the atom d) The mass of t ...
... Which of the following conclusions can be drawn based on the outcome of Rutherford’s experiment (scattering of a-particles by gold foil)?* a) Electrons are rotating in circles b) Electrons are not moving at all c) The volume of the nucleus is much smaller than the volume of the atom d) The mass of t ...
Chap. 4 AQUEOUS RXNS O
... 6. The sum of all O.N. in a neutral compound is 0, otherwise ΣO.N. = ion charge ...
... 6. The sum of all O.N. in a neutral compound is 0, otherwise ΣO.N. = ion charge ...
Multielectron Atoms * The Independent Particle Approximation
... • Ground state: two electrons in 1s state (spin up/down) • Screening of each electron by the other • This results on a relatively large ionization energy (energy to remove an electron from a neutral atom) of 24.6 eV • Also large excitation energy (E2s-E1s) = 19.8 eV vs. 10.2 eV for H • Chemically in ...
... • Ground state: two electrons in 1s state (spin up/down) • Screening of each electron by the other • This results on a relatively large ionization energy (energy to remove an electron from a neutral atom) of 24.6 eV • Also large excitation energy (E2s-E1s) = 19.8 eV vs. 10.2 eV for H • Chemically in ...
Chemistry Terms
... endothermic (reaction) A chemical reaction that requires an input of energy to drive it. exothermic (reaction) A chemical reaction in which energy is released to the environment. ionic bond A bond between atoms in which an electron from one atom leaves and resides in the other shell of the other ato ...
... endothermic (reaction) A chemical reaction that requires an input of energy to drive it. exothermic (reaction) A chemical reaction in which energy is released to the environment. ionic bond A bond between atoms in which an electron from one atom leaves and resides in the other shell of the other ato ...
Ch 2.1 and 2.2 Review
... Solution is homogeneous (evenly distributed to look like one substance). Suspension is heterogeneous (particles do not evenly mix and can be seen in the liquid. ...
... Solution is homogeneous (evenly distributed to look like one substance). Suspension is heterogeneous (particles do not evenly mix and can be seen in the liquid. ...
The format of this test is MULTIPLE CHOICE
... 1. __Condensation___ occurs when a gas becomes a liquid. 2. All matter is made up of tiny particles called __atoms___. 3. When a solid becomes a liquid, _melting_____ occurs. 4. An _element_____ is made up of only one type of atom. 5. __freezing___ changes a liquid into a solid. 6. A mixture is made ...
... 1. __Condensation___ occurs when a gas becomes a liquid. 2. All matter is made up of tiny particles called __atoms___. 3. When a solid becomes a liquid, _melting_____ occurs. 4. An _element_____ is made up of only one type of atom. 5. __freezing___ changes a liquid into a solid. 6. A mixture is made ...
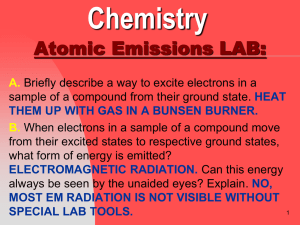
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
2A Final Exam Review Worksheet
... o Isotope = occurs when the number of neutrons vary for a particular element. The number of protons do not vary. o Given any two of these variables, be able to solve for the third: weighted average atomic mass, isotopic mass, fractional abundance Counting protons, neutrons, and electrons in elements ...
... o Isotope = occurs when the number of neutrons vary for a particular element. The number of protons do not vary. o Given any two of these variables, be able to solve for the third: weighted average atomic mass, isotopic mass, fractional abundance Counting protons, neutrons, and electrons in elements ...
ppt Sc10 Review Notes
... D. Ions ions are particles or groups of particles that have a net charge (either positive or negative) neutral atoms are unstable if their valence level is not full ...
... D. Ions ions are particles or groups of particles that have a net charge (either positive or negative) neutral atoms are unstable if their valence level is not full ...
Covalent Bonds - WordPress.com
... • In a nonpolar covalent bond, the atoms share the electron equally • A covalent bond between two atoms of the same element nonpolar :H-H ;Cl-Cl …. • A covalent bond between atoms that have similar electronegativities,the bond of CH4 are nonpolar • In a polar covalent bond, one atom is more electro ...
... • In a nonpolar covalent bond, the atoms share the electron equally • A covalent bond between two atoms of the same element nonpolar :H-H ;Cl-Cl …. • A covalent bond between atoms that have similar electronegativities,the bond of CH4 are nonpolar • In a polar covalent bond, one atom is more electro ...
final exam review chapter 1-4
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...
AP Chemistry Summer Assignment - Belle Vernon Area School District
... 1. Complete the practice problems in this packet. You may use the resources listed below as well as any notes/worksheets from Accel. Chem that you may have. 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on th ...
... 1. Complete the practice problems in this packet. You may use the resources listed below as well as any notes/worksheets from Accel. Chem that you may have. 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on th ...
Solutions - Seattle Central
... compound. A molecule of a compound is the smallest particle that has the specific properties of the compound CH4 (methane). ...
... compound. A molecule of a compound is the smallest particle that has the specific properties of the compound CH4 (methane). ...