The Lippmann-Schwinger equation reads ψk(x) = φk(x) + ∫ dx G0(x
... V . That is, ψk is replaced by φk ; • if the relative motion is treated by means of plane waves we have the plane-wave Born approximation (PWBA); • an alternative choice is when H0 includes part of the interaction (an optical potential U ). Then the relative motion part of the wavefunction is a ”dis ...
... V . That is, ψk is replaced by φk ; • if the relative motion is treated by means of plane waves we have the plane-wave Born approximation (PWBA); • an alternative choice is when H0 includes part of the interaction (an optical potential U ). Then the relative motion part of the wavefunction is a ”dis ...
... the Lagrangian approach [14, 10, 11, 16, 4]. The Hamiltonian formalism gives rise to the canonical quantization, while the Lagrangian approach is used in the path-integral quantization. Usually, in classical mechanics, there is a transformation that relates these two approaches. However, for a repar ...
Exam 1 as pdf
... (a) (5) What is the potential energy Vt due to this force, as a function of time, with Vt = 0 at x = 0 ? (b) (15) Using time-dependent perturbation theory to first order, calculate the probability of finding the oscillator in its first excited state for t > 0 . Give your answer in terms of τ , F0 , ...
... (a) (5) What is the potential energy Vt due to this force, as a function of time, with Vt = 0 at x = 0 ? (b) (15) Using time-dependent perturbation theory to first order, calculate the probability of finding the oscillator in its first excited state for t > 0 . Give your answer in terms of τ , F0 , ...
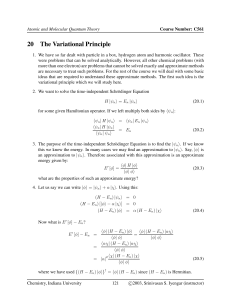
453 Introduction to Quantum Mechanics (Winter 2005)
... Assuming that the number of free electrons per unit area is σ, calculate the Fermi energy for electrons in a two-dimensional infinite square well. 7. The most prominent feature of the hydrogen spectrum in the visible region is the red Balmer line, coming from the transition n = 3 to n = 2. i) Determ ...
... Assuming that the number of free electrons per unit area is σ, calculate the Fermi energy for electrons in a two-dimensional infinite square well. 7. The most prominent feature of the hydrogen spectrum in the visible region is the red Balmer line, coming from the transition n = 3 to n = 2. i) Determ ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
... 2. Prove explicitly that the momentum operator is a self-adjoint operator. 3. Write down the ground state energy eigenfunction of a simple harmonic oscillator? Sketch its graph. 4. Define the parity operator by its effect on a wave function. What are its eigenvalues? 5. If A is any Hermitian operato ...
Helium Atom
... This observation cannot be described by simple concept from hydrogen atom. When more than one electron is present, the Hamiltonian for an atom in free space becomes ...
... This observation cannot be described by simple concept from hydrogen atom. When more than one electron is present, the Hamiltonian for an atom in free space becomes ...
Internal Degrees of Freedom of Molecules ( + problem 33)
... is the effective potential energy equal to the sum of bare potential energy, V (R), (Coulomb repulsion between nuclei) and the electronic energy as a function of R. Note that it is entirely due to the (R) attractive term Eel that nuclei bind into a molecule. The effective adiabatic potential Veff (R ...
... is the effective potential energy equal to the sum of bare potential energy, V (R), (Coulomb repulsion between nuclei) and the electronic energy as a function of R. Note that it is entirely due to the (R) attractive term Eel that nuclei bind into a molecule. The effective adiabatic potential Veff (R ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 18. Illustrate the Pauli Exclusion Principle for the ground state of He atom. 19. At what distance from the nucleus is the probability of finding the electron a maximum for a 1S electron in hydrogen? 20. While the order is the same for both C3v and C3h point groups, their classes are different reaso ...
... 18. Illustrate the Pauli Exclusion Principle for the ground state of He atom. 19. At what distance from the nucleus is the probability of finding the electron a maximum for a 1S electron in hydrogen? 20. While the order is the same for both C3v and C3h point groups, their classes are different reaso ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... order correction to the ground state energy of an anharmonic oscillator of mass m and angular ngular frequency ω subjected to a potential. ...
... order correction to the ground state energy of an anharmonic oscillator of mass m and angular ngular frequency ω subjected to a potential. ...
Supersymmetric Quantum Mechanics and Reflectionless Potentials
... and force terms – Has several interesting consequences such as • Every fundamental particle has a super particle (matches bosons to fermionic super partners and vice versa ...
... and force terms – Has several interesting consequences such as • Every fundamental particle has a super particle (matches bosons to fermionic super partners and vice versa ...
Lecture 12
... lower energy. It is also called an annihilation operator, because it removes one quantum of energy �ω from the system. Similarly it is straightforward to show that Ĥ↠|n� = (En + �ω)↠|n� , which says that ↠|n� is an eigenfunction of Ĥ belonging to the eigenvalue (En + �ω), unless ↠|n� ≡ ...
... lower energy. It is also called an annihilation operator, because it removes one quantum of energy �ω from the system. Similarly it is straightforward to show that Ĥ↠|n� = (En + �ω)↠|n� , which says that ↠|n� is an eigenfunction of Ĥ belonging to the eigenvalue (En + �ω), unless ↠|n� ≡ ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 11. Derive the Schroedinger time-independent wave equation from the time-dependent one. 12. What is a hermitian operator and its significance? Show that eigen functions corresponding to two different eigen values of a hermitian operator are orthogonal. 13. Show that in spherical polar coordinates th ...
... 11. Derive the Schroedinger time-independent wave equation from the time-dependent one. 12. What is a hermitian operator and its significance? Show that eigen functions corresponding to two different eigen values of a hermitian operator are orthogonal. 13. Show that in spherical polar coordinates th ...
Supplment to Chapter 24: Energy Levels of a Free
... Supplment to Chapter 24: Energy Levels of a Free Particle in a Box Section 24.1’s derivation of the equation of state of a gas of free, spin-1/2 fermions assumed some elementary and standard facts about the energy levels of single quantum mechanical particle confined to a box. For completeness, we r ...
... Supplment to Chapter 24: Energy Levels of a Free Particle in a Box Section 24.1’s derivation of the equation of state of a gas of free, spin-1/2 fermions assumed some elementary and standard facts about the energy levels of single quantum mechanical particle confined to a box. For completeness, we r ...