Quantum Harmonic Oscillator
... "quantized", and may only take the discrete values of times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not ...
... "quantized", and may only take the discrete values of times 1/2, 3/2, 5/2, and so forth. This is a feature of many quantum mechanical systems. In the following section on ladder operators, we will engage in a more detailed examination of this phenomenon. Secondly, the lowest achievable energy is not ...
Problem set 2
... 2. Give an example of a state with zero angular momentum ~L = 0 (located at a finite distance from the origin and with finite energy E < 0) for such a particle. h2i 3. Write the Hamiltonian and Hamilton’s equations in spherical coordinates for a particle with zero angular momentum in the above poten ...
... 2. Give an example of a state with zero angular momentum ~L = 0 (located at a finite distance from the origin and with finite energy E < 0) for such a particle. h2i 3. Write the Hamiltonian and Hamilton’s equations in spherical coordinates for a particle with zero angular momentum in the above poten ...
6. Quantum Mechanics II
... is ~ a square well. The potential barrier at the nuclear radius is several times greater than the energy of an alpha particle. In quantum mechanics, however, the alpha particle can tunnel through the barrier. This is radioactive decay! ...
... is ~ a square well. The potential barrier at the nuclear radius is several times greater than the energy of an alpha particle. In quantum mechanics, however, the alpha particle can tunnel through the barrier. This is radioactive decay! ...
Canonical quantization of scalar fields
... are arbitrary functions of , and is a function of |k| (introduced for later convenience) if we tried to interpret as a quantum wave function, the second term would represent contributions with negative energy to the wave function! ...
... are arbitrary functions of , and is a function of |k| (introduced for later convenience) if we tried to interpret as a quantum wave function, the second term would represent contributions with negative energy to the wave function! ...
IB HL Physics More Problems on Quantum and Nuclear Physics_
... palladium nucleus. It then moves back along the path from which it came as shown in the diagram below. palladium nucleus -particle ...
... palladium nucleus. It then moves back along the path from which it came as shown in the diagram below. palladium nucleus -particle ...
Chemistry 2000 Review: quantum mechanics of
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
Marvin_Weinstein
... required, just a choice of coupling constant. Question: What do we hold fixed ? My choice is the energy of the zero momentum two-particle state. This immediately shows why this theory is trivial in four dimensions. ...
... required, just a choice of coupling constant. Question: What do we hold fixed ? My choice is the energy of the zero momentum two-particle state. This immediately shows why this theory is trivial in four dimensions. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... standard integral 0 exp(-bx2) dx = (1/2)(/b)1/2.] 19. Define or explain the three parts that make an atomic term symbol and formulate the term symbols for the ground state configuration of F atom. 20. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (H ...
... standard integral 0 exp(-bx2) dx = (1/2)(/b)1/2.] 19. Define or explain the three parts that make an atomic term symbol and formulate the term symbols for the ground state configuration of F atom. 20. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (H ...
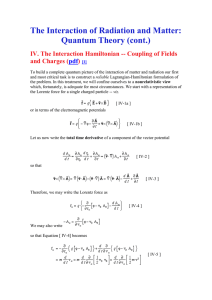
The Interaction of Radiation and Matter: Quantum
... and, using the cannonically conjugate momenta associated Equation [ IV-8 ] -- i.e., ...
... and, using the cannonically conjugate momenta associated Equation [ IV-8 ] -- i.e., ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 2) In what way is the orthonormal property of eigenfunctions belonging to discrete set of eigenvalues different from those of continuous set? 3) Define parity operator. What is its action on a wave function ψ ( r, θ, φ)? 4) What is a rigid rotator and what are its energy eigenvalues? 5) Expand an ar ...
... 2) In what way is the orthonormal property of eigenfunctions belonging to discrete set of eigenvalues different from those of continuous set? 3) Define parity operator. What is its action on a wave function ψ ( r, θ, φ)? 4) What is a rigid rotator and what are its energy eigenvalues? 5) Expand an ar ...
Review Sheet
... Kinetic vs. potential energy Enthalpy (H) Measuring heat (q) Specific heat capacity Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations ...
... Kinetic vs. potential energy Enthalpy (H) Measuring heat (q) Specific heat capacity Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations ...
3.1 The correspondence principle
... The fraction of each Eigenvector to the sum of all states will change generally as a function of time. ⇒ The state of a system will normally change in time. REMARKS: • In physics the formalism of energy is much more fundamental than the formalism of using forces. • All forces which apply to an elect ...
... The fraction of each Eigenvector to the sum of all states will change generally as a function of time. ⇒ The state of a system will normally change in time. REMARKS: • In physics the formalism of energy is much more fundamental than the formalism of using forces. • All forces which apply to an elect ...
Introduction to Electromagnetism
... Time and space are NOT absolute, but their interrelatedness shows up only at very high speeds, where moving objects contract and ...
... Time and space are NOT absolute, but their interrelatedness shows up only at very high speeds, where moving objects contract and ...
A1980KM40500001
... paper shows that the Hamiltonian can be transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampt ...
... paper shows that the Hamiltonian can be transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampt ...