Hydrogen Atom in Spherical Coordinates (III) Eigenenergies of one
... Angular Functions : Spherical Harmonics ...
... Angular Functions : Spherical Harmonics ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... The value of Z that minimize E can be interpreted as an effective charge. That fact that Z comes out to be less than 2 reflects the fact that each electron partially screens the nucleus from the other , so that net effective nuclear charge is reduced from 2 to 27/16 (1.68). ...
... The value of Z that minimize E can be interpreted as an effective charge. That fact that Z comes out to be less than 2 reflects the fact that each electron partially screens the nucleus from the other , so that net effective nuclear charge is reduced from 2 to 27/16 (1.68). ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 /1.00-4.00
... Part-C ANSWER ANY FOUR QUESTIONS (4 x 10 = 40) 23. (a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on the angles θ and φ (b) In the Compton experiment, a beam of ...
... Part-C ANSWER ANY FOUR QUESTIONS (4 x 10 = 40) 23. (a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on the angles θ and φ (b) In the Compton experiment, a beam of ...
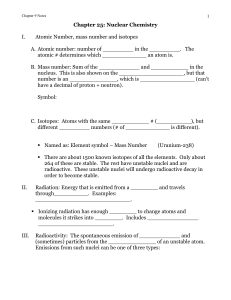
Chapter 9: Nuclear Chemistry
... ________ penetrating than alpha particles. There are also ______ particles (_______________) that are identical to electrons but have a ____ charge. Symbols: C. Gamma ( ) emission: Pure _____________________ energy; more energetic than _____________. Travels at the speed of light. Has no _________ o ...
... ________ penetrating than alpha particles. There are also ______ particles (_______________) that are identical to electrons but have a ____ charge. Symbols: C. Gamma ( ) emission: Pure _____________________ energy; more energetic than _____________. Travels at the speed of light. Has no _________ o ...
The Hydrogen Atom - Valdosta State University
... vary since real atoms don’t have fixed distances between nuclei and electrons. 2. Use separation of variables to pull out part already solved for rigid rotor (angular part). 3. Show solution for radial part (r dependent part). E energy will depend only on the n quantum number (and not m or l). Final ...
... vary since real atoms don’t have fixed distances between nuclei and electrons. 2. Use separation of variables to pull out part already solved for rigid rotor (angular part). 3. Show solution for radial part (r dependent part). E energy will depend only on the n quantum number (and not m or l). Final ...
Document
... Annihilate the quantum whereas the negative energy component create the quantum. This quantum is called a particle of positive energy. ...
... Annihilate the quantum whereas the negative energy component create the quantum. This quantum is called a particle of positive energy. ...
Physics 7910: HW # 03.
... ~r as a function of two-dimensional position Describe also the magnetic order that obtains, by explicit result for S vector r. 3. Holstein-Primakoff representation of the spin operator S is given by r r ...
... ~r as a function of two-dimensional position Describe also the magnetic order that obtains, by explicit result for S vector r. 3. Holstein-Primakoff representation of the spin operator S is given by r r ...
$doc.title
... Q and f are canonical variables see e.g.: Goldstein, Classical Mechanics, Chapter 8, Hamilton Equations of Motion ...
... Q and f are canonical variables see e.g.: Goldstein, Classical Mechanics, Chapter 8, Hamilton Equations of Motion ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 11. a) If a particle’s kinetic energy is equal to its rest mass energy, what is its speed? b) Obtain the relation between the relativistic energy and momentum. (3 ½ +4) 12. Explain how the components of electric field transform as viewed from another inertial frame. 13. Outline the wave mechanical p ...
... 11. a) If a particle’s kinetic energy is equal to its rest mass energy, what is its speed? b) Obtain the relation between the relativistic energy and momentum. (3 ½ +4) 12. Explain how the components of electric field transform as viewed from another inertial frame. 13. Outline the wave mechanical p ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of event B are ( x B, 0, 0, t B). Assuming the interval between them is time like, find the velocity of the system in which they occur at same place. 17. Establish the covariant formulation of Maxwell’s equations. 18. Discuss t ...
... (b) The coordinates of event A are ( x A, 0, 0, t A) and the coordinates of event B are ( x B, 0, 0, t B). Assuming the interval between them is time like, find the velocity of the system in which they occur at same place. 17. Establish the covariant formulation of Maxwell’s equations. 18. Discuss t ...
Quantum Mechanics: PHL555 Tutorial 2
... part of the Hamiltonian represents the interaction with the magnetic field. We have neglected the effects due to spin angular momentum of the electron . Treat H 1 as a perturbation and show s(l 0) states are not split , where as p(l 1) states are split into three states separated by the energy i ...
... part of the Hamiltonian represents the interaction with the magnetic field. We have neglected the effects due to spin angular momentum of the electron . Treat H 1 as a perturbation and show s(l 0) states are not split , where as p(l 1) states are split into three states separated by the energy i ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Simple Harmonic Oscillator models in terms of its wave function and its energy. 14. Explain with an example: (a) Bohr's Correspondence Principle (b) BornOppenheimer Approximation 15. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (HAA + HAB) / (1+SAB) w ...
... Simple Harmonic Oscillator models in terms of its wave function and its energy. 14. Explain with an example: (a) Bohr's Correspondence Principle (b) BornOppenheimer Approximation 15. In solving the H2+ problem using the LCAO method, the lowest energy obtained is given by E+ = (HAA + HAB) / (1+SAB) w ...
Group Problems #27 - Solutions Wednesday, November 2 Problem 1
... So if we constrain our measurement to a particular value of position (x), then we will measure a spread in kinetic energy values when we repeat the measurement many times on a similarly prepared system. (d) What is the expectation value for the momentum of a particle in the ground state of the harmo ...
... So if we constrain our measurement to a particular value of position (x), then we will measure a spread in kinetic energy values when we repeat the measurement many times on a similarly prepared system. (d) What is the expectation value for the momentum of a particle in the ground state of the harmo ...