UVM Physics MS: Comprehensive Exam Date: Saturday January 11, 2013 Time:
... (b) Impose the correct boundary conditions at the origin. Show that in addition to the wavefunction continuity equation ψ(+0) = ψ(−0), there is also a condition on the wave-function derivative (which experiences a jump): ψ 0 (+0) − ψ 0 (−0) = − ...
... (b) Impose the correct boundary conditions at the origin. Show that in addition to the wavefunction continuity equation ψ(+0) = ψ(−0), there is also a condition on the wave-function derivative (which experiences a jump): ψ 0 (+0) − ψ 0 (−0) = − ...
collective states of 2d electron-hole system under the influence of
... Key words: magnetoexciton, Bose-Einstein condensation, electron-hole liquid This study is concerned with a two-dimensional (2D) electron–hole system in an ideal symmetric 2D layer in a strong perpendicular magnetic field with special attention devoted to the Rashba spin–orbit coupling. The electric ...
... Key words: magnetoexciton, Bose-Einstein condensation, electron-hole liquid This study is concerned with a two-dimensional (2D) electron–hole system in an ideal symmetric 2D layer in a strong perpendicular magnetic field with special attention devoted to the Rashba spin–orbit coupling. The electric ...
Types of Radiation
... Spontaneous nuclear change to attain good n/p ratio (high stability, low energy state). Form a new kind of atom. Each isotope or nuclide decays in a certain manner to get a better n/p ratio. The decay mode is named for the particle emitted. See Table N. ...
... Spontaneous nuclear change to attain good n/p ratio (high stability, low energy state). Form a new kind of atom. Each isotope or nuclide decays in a certain manner to get a better n/p ratio. The decay mode is named for the particle emitted. See Table N. ...
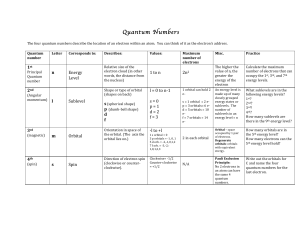
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... Again, it would be a good idea to read this section for ideas, but I won't test you over it. The main idea is that electrons and nuclei orbit each other. The much more massive nucleus moves very little, just as the earth does most of the orbiting around the sun. However, on the atomic scale, the cor ...
... Again, it would be a good idea to read this section for ideas, but I won't test you over it. The main idea is that electrons and nuclei orbit each other. The much more massive nucleus moves very little, just as the earth does most of the orbiting around the sun. However, on the atomic scale, the cor ...
Chemistry I Honors – Semester Exam Review – Fall 2000
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
1 - M*W
... gas. Which of the following represents the reactants in this reaction? a) Magnesium and magnesium chloride b) Hydrochloric acid and hydrogen gas c) Magnesium and hydrochloric acid d) Magnesium chloride and hydrogen gas 50) The equation E=mc2 shows that a) Chemical reactions are either exothermic or ...
... gas. Which of the following represents the reactants in this reaction? a) Magnesium and magnesium chloride b) Hydrochloric acid and hydrogen gas c) Magnesium and hydrochloric acid d) Magnesium chloride and hydrogen gas 50) The equation E=mc2 shows that a) Chemical reactions are either exothermic or ...
Dr David M. Benoit (david.benoit@uni
... , are the energy levels (energy of molecular orbitals, for example) and the eigenfunctions, , represent the allowed steadystate wave functions for the system ...
... , are the energy levels (energy of molecular orbitals, for example) and the eigenfunctions, , represent the allowed steadystate wave functions for the system ...
Lectures 1-2
... atomic orbitals from which they were derived. If the total energy of the electrons is lower using molecular orbitals (the middle column), the molecule forms. If the total energy of the electrons is lower using atomic orbitals (the two outside columns), no molecule is formed. To fill a molecular orbi ...
... atomic orbitals from which they were derived. If the total energy of the electrons is lower using molecular orbitals (the middle column), the molecule forms. If the total energy of the electrons is lower using atomic orbitals (the two outside columns), no molecule is formed. To fill a molecular orbi ...
Rotation Vibration Spectrum of the HCl Molecule
... molecule. The equilibrium bond length re for Because the nuclei are much heavier than the the bonding state is also shown. The zero of electrons, they move much more slowly. To potential energy has been chosen to be that of a good approximation the nuclei can be con- two isolated atoms, r = ∞. sider ...
... molecule. The equilibrium bond length re for Because the nuclei are much heavier than the the bonding state is also shown. The zero of electrons, they move much more slowly. To potential energy has been chosen to be that of a good approximation the nuclei can be con- two isolated atoms, r = ∞. sider ...
Exercises to Quantum Mechanics FYSN17
... Exercise 1: First-order correction of energy Aim: Learning how to apply the formalism of stationary perturbation theory ...
... Exercise 1: First-order correction of energy Aim: Learning how to apply the formalism of stationary perturbation theory ...
NUCLEAR PHYSICS
... momentum is a property of a physical system that is a constant of motion[1] (is time-independent and well-defined) in two situations: (i) The system experiences a spherically symmetric potential field. (ii) The system moves (in quantum mechanical sense) in isotropic space. In both cases the angular ...
... momentum is a property of a physical system that is a constant of motion[1] (is time-independent and well-defined) in two situations: (i) The system experiences a spherically symmetric potential field. (ii) The system moves (in quantum mechanical sense) in isotropic space. In both cases the angular ...
Population Analysis
... that when charge is near an atom it "belongs" to that atom and the mathematically convenient population analysis has spawned numerous attempts at defining a more realistic partitioning. The problem is tied to the inherent ambiguity of partitioning a continuously varying charge distribution among a f ...
... that when charge is near an atom it "belongs" to that atom and the mathematically convenient population analysis has spawned numerous attempts at defining a more realistic partitioning. The problem is tied to the inherent ambiguity of partitioning a continuously varying charge distribution among a f ...
Lecture 4: Nuclear Energy Generation
... Magic numbers: nuclear physics Nuclear physics looks at magic numbers slightly differently: The liquid drop model is a good guide on the overall behavior of the average binding energy per nucleon. However, detailed structures are not accounted for. There is a set of magic numbers and nuclei with th ...
... Magic numbers: nuclear physics Nuclear physics looks at magic numbers slightly differently: The liquid drop model is a good guide on the overall behavior of the average binding energy per nucleon. However, detailed structures are not accounted for. There is a set of magic numbers and nuclei with th ...