Description of NOVA`s The Fabric of the Cosmos “Quantum Leap
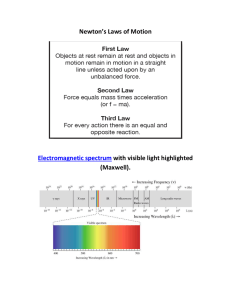
... - Niels Bohr explained spectral lines as the energy given off by electrons jumping between an atom’s orbitals. But the electrons don’t travel across the space—they show up in a different orbit without physically traveling through the “between” space. What makes the quantum leap so strange is that th ...
... - Niels Bohr explained spectral lines as the energy given off by electrons jumping between an atom’s orbitals. But the electrons don’t travel across the space—they show up in a different orbit without physically traveling through the “between” space. What makes the quantum leap so strange is that th ...
5.0. Wave Mechanics
... The particle-wave duality is best described by the wave mechanical formulism of quantum theory invented by Schrodinger. Thus, the state of a “particle” is represented by a (complex) wave function x,t so that the probability of finding the particle in an infinitesimal volume d 3 x about x at ti ...
... The particle-wave duality is best described by the wave mechanical formulism of quantum theory invented by Schrodinger. Thus, the state of a “particle” is represented by a (complex) wave function x,t so that the probability of finding the particle in an infinitesimal volume d 3 x about x at ti ...
You are going to read the chapter at home.
... Completeness: We can expand the Nparticle wave function as a product of single-particle wave functions __ ...
... Completeness: We can expand the Nparticle wave function as a product of single-particle wave functions __ ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
research project #1 - Soudan Underground Laboratory
... MN. The near detector and particle accelerator (beginning of beam) are in Fermilab, while the far detector is in Soudan. ...
... MN. The near detector and particle accelerator (beginning of beam) are in Fermilab, while the far detector is in Soudan. ...