Physics 1906 JOSEPH JOHN THOMSON
... tube through which an electric discharge was passing. When an electric discharge is sent through a highly exhausted tube, the sides of the tube glow with a vivid green phosphorescence. That this is due to something proceeding in straight lines from the cathode - the electrode where the negative elec ...
... tube through which an electric discharge was passing. When an electric discharge is sent through a highly exhausted tube, the sides of the tube glow with a vivid green phosphorescence. That this is due to something proceeding in straight lines from the cathode - the electrode where the negative elec ...
Properties, Statistics and the Identity of Quantum Particles
... individual objects 2) It is a widespread belief that quantum theory refutes the intuition, as it is a theory of non-individuals • ‘Received View’, dating back to (some of) the founding fathers of the theory - e.g., Born, Heisenberg • «It is impossible for either of these individuals to retain his id ...
... individual objects 2) It is a widespread belief that quantum theory refutes the intuition, as it is a theory of non-individuals • ‘Received View’, dating back to (some of) the founding fathers of the theory - e.g., Born, Heisenberg • «It is impossible for either of these individuals to retain his id ...
Quantum Mechanics 1 - University of Birmingham
... we go to the atomic regime (where E and m are very small) – then we need to consider Quantum Mechanics. • CM also fails when velocity is very large (as v c), due to relativistic effects. ...
... we go to the atomic regime (where E and m are very small) – then we need to consider Quantum Mechanics. • CM also fails when velocity is very large (as v c), due to relativistic effects. ...
Solving the Helium Atom
... Before we get started, however, we will briefly review the principles of quantum mechanics. Particles do not actually have specific positions or many other qualities we attribute to macroscopic objects. Rather, they are described by “wave functions” which determine the probability of a particle bein ...
... Before we get started, however, we will briefly review the principles of quantum mechanics. Particles do not actually have specific positions or many other qualities we attribute to macroscopic objects. Rather, they are described by “wave functions” which determine the probability of a particle bein ...
Matter Unit Study Guide Phases of Matter
... Mixtures of solids can be separated based on observable properties of their parts such as: size, color, or shape. Some mixtures are not as easy to separate, but since each substance mixed keeps its identity, it can be separated using its physical properties. Circle the 7 words below that are physica ...
... Mixtures of solids can be separated based on observable properties of their parts such as: size, color, or shape. Some mixtures are not as easy to separate, but since each substance mixed keeps its identity, it can be separated using its physical properties. Circle the 7 words below that are physica ...
Astronomy 253 (Spring 2016) Collisions and Transport
... Consider a test particle – let it be an electron – interacting with background field particles – let them be protons for the moment. The test particle travels at speed ve , and we treat protons as being at rest (because their velocities are typically much smaller). When the electron passes by a sing ...
... Consider a test particle – let it be an electron – interacting with background field particles – let them be protons for the moment. The test particle travels at speed ve , and we treat protons as being at rest (because their velocities are typically much smaller). When the electron passes by a sing ...
Mechanics 1: Work, Power and Kinetic Energy
... Example. Suppose a particle of mass m moves under the influence of a force field along a space curve whose position vector is given by: r = (2t3 + t)i + (3t4 − t2 + 8)j − 12t2 k. Find the instantaneous power applied to the particle by the force field. ...
... Example. Suppose a particle of mass m moves under the influence of a force field along a space curve whose position vector is given by: r = (2t3 + t)i + (3t4 − t2 + 8)j − 12t2 k. Find the instantaneous power applied to the particle by the force field. ...
Circuit Elements
... Called an elementary particle, since it seems to have no sub-particles Has mass of approx. 1/1836 of a proton Yet electrons have some properties of particles AND waves ...
... Called an elementary particle, since it seems to have no sub-particles Has mass of approx. 1/1836 of a proton Yet electrons have some properties of particles AND waves ...
PPT
... A Theory of Everything, expressed in a single equation, accounting for all four of the above theories. The Superstring Theory is the latest theory attempting to realize this objective. ...
... A Theory of Everything, expressed in a single equation, accounting for all four of the above theories. The Superstring Theory is the latest theory attempting to realize this objective. ...
IPC Semester Exam Review – Chemistry Topics
... 51. Proposed the existence of a dense, +charged nucleus. 52. Discovered radioactivity. 53. Proposed the existence of –charged electrons. 54. Proposed the existence of neutrons. 55. Developed the “electron cloud” model of the atom. 56. Proposed that electrons travel in circular orbits. 57. Draw atomi ...
... 51. Proposed the existence of a dense, +charged nucleus. 52. Discovered radioactivity. 53. Proposed the existence of –charged electrons. 54. Proposed the existence of neutrons. 55. Developed the “electron cloud” model of the atom. 56. Proposed that electrons travel in circular orbits. 57. Draw atomi ...
Millikan`s Oil Drop Experiment
... O The force on a spherical object can be calculated using Stoke’s Law: F=6πηrv η=coeff of viscosity of fluid, r=radius of object v=velocity of object, π=Pi ...
... O The force on a spherical object can be calculated using Stoke’s Law: F=6πηrv η=coeff of viscosity of fluid, r=radius of object v=velocity of object, π=Pi ...
v - Purdue Physics
... q vBsin 90 m R (high q – small R, large m large R) Applications: find p, e/m, separate particles (including 238U and 235U (natural abundance 0.7%) isotopes ...
... q vBsin 90 m R (high q – small R, large m large R) Applications: find p, e/m, separate particles (including 238U and 235U (natural abundance 0.7%) isotopes ...
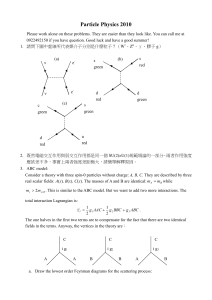
Particle Physics
... needed. You can drop all the multiplicative constants. Comment: This is the result for a structure-less scattering. Compare it to the answers in 3c, 3d where there is a propagating mediating particle. From the experimental data, we can tell which the case is. b. Consider the process A( p1 ) B( p2 ...
... needed. You can drop all the multiplicative constants. Comment: This is the result for a structure-less scattering. Compare it to the answers in 3c, 3d where there is a propagating mediating particle. From the experimental data, we can tell which the case is. b. Consider the process A( p1 ) B( p2 ...
PHYS 1443 – Section 501 Lecture #1
... Elementary Particles • How do these particles interact?? – All particles, including photons and neutrinos, participate in gravitational interactions – Photons can interact electromagnetically with any particles with electric charge – All charged leptons participate in both EM and weak interactions ...
... Elementary Particles • How do these particles interact?? – All particles, including photons and neutrinos, participate in gravitational interactions – Photons can interact electromagnetically with any particles with electric charge – All charged leptons participate in both EM and weak interactions ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.