RAD 107 HOMEWORK 4
... 2. Because each chemical element corresponds to precisely one value of the atomic number, nuclides can also be identified by the symbol for the element followed by the mass number (i.e. without also specifying the atomic number). For example, instead of 146 C we can write C-14 or Carbon-14. Write ea ...
... 2. Because each chemical element corresponds to precisely one value of the atomic number, nuclides can also be identified by the symbol for the element followed by the mass number (i.e. without also specifying the atomic number). For example, instead of 146 C we can write C-14 or Carbon-14. Write ea ...
Student Notes Chapter 17
... have different quantum states as a result. This idea leads to the Pauli exclusion principle that no two particles, electrons in atoms or protons and neutrons in nuclei, ever share the same quantum state. Photons do exactly the opposite. The amplitudes of two identical photons at the same point in sp ...
... have different quantum states as a result. This idea leads to the Pauli exclusion principle that no two particles, electrons in atoms or protons and neutrons in nuclei, ever share the same quantum state. Photons do exactly the opposite. The amplitudes of two identical photons at the same point in sp ...
Worksheet - 1 - International Indian School, Riyadh
... 7. How many unpaired electrons are present in N? Name the principle which explains the presence of these unpaired electrons. 2 or more marks Question: 8. Write a short note on Plank’s Quantum theory. 9. Calculate the wavelength of an electron that has been accelerated in a particle accelerator throu ...
... 7. How many unpaired electrons are present in N? Name the principle which explains the presence of these unpaired electrons. 2 or more marks Question: 8. Write a short note on Plank’s Quantum theory. 9. Calculate the wavelength of an electron that has been accelerated in a particle accelerator throu ...
Solutions - Stanford University
... (d) Use the result of part (c) to conclude that if we start in a state with eigenvalue N = 2, while H is non-diagonal in an infinite-dimensional Hilbert space, we need only worry about the dynamics in a subspace of dimension 3. Give a simple basis for this subspace. Solution: The states with N = 2 h ...
... (d) Use the result of part (c) to conclude that if we start in a state with eigenvalue N = 2, while H is non-diagonal in an infinite-dimensional Hilbert space, we need only worry about the dynamics in a subspace of dimension 3. Give a simple basis for this subspace. Solution: The states with N = 2 h ...
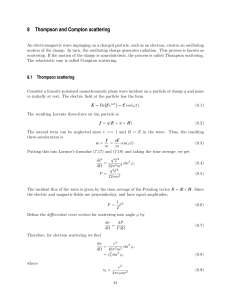
For a “black body” - The University of Sheffield
... thermal radiation doesn’t require a “medium” •So, for example, heat can reach Earth from the Sun through millions of kilometres of empty space. •Rate at which an object, surface area A, temperature T, radiates energy is given by Stefan’s Law ...
... thermal radiation doesn’t require a “medium” •So, for example, heat can reach Earth from the Sun through millions of kilometres of empty space. •Rate at which an object, surface area A, temperature T, radiates energy is given by Stefan’s Law ...
Nick-Evans
... We also are using this to better understand gauge theories (QCD?) * confinement (hadronization) ...
... We also are using this to better understand gauge theories (QCD?) * confinement (hadronization) ...
ATOMIC NUMBER…it can be found on the periodic table
... the periodic table and are mostly inert (non-reactive). (NOBEL GASSES…these are the elements that don’t react because their outer shells are filled up with electrons! To quote Bruno Mars: They are beautiful just the way they are!) 14. These are elements on the periodic table that are both metals and ...
... the periodic table and are mostly inert (non-reactive). (NOBEL GASSES…these are the elements that don’t react because their outer shells are filled up with electrons! To quote Bruno Mars: They are beautiful just the way they are!) 14. These are elements on the periodic table that are both metals and ...
Chapter 7
... -an electron cloud: an “envelope” that is very large in volume, light compared to the nucleus, and negatively charged. Because the protons in an atom’s nucleus account for less than half the mass calculated for the nucleus, Rutherford inferred that the nucleus must also contain additional, uncharged ...
... -an electron cloud: an “envelope” that is very large in volume, light compared to the nucleus, and negatively charged. Because the protons in an atom’s nucleus account for less than half the mass calculated for the nucleus, Rutherford inferred that the nucleus must also contain additional, uncharged ...
A Physical Model for Atoms and Nuclei—Part 3
... Bohr’s postulates were very radical. They assumed that some electromagnetic laws, such as Coulomb’s force law held on the microscopic scale, but not Ampere’s law or Faraday’s law. Thus the laws of physics were assumed to be different on the microscopic scale than on the macroscopic scale. Also, Bohr ...
... Bohr’s postulates were very radical. They assumed that some electromagnetic laws, such as Coulomb’s force law held on the microscopic scale, but not Ampere’s law or Faraday’s law. Thus the laws of physics were assumed to be different on the microscopic scale than on the macroscopic scale. Also, Bohr ...
idenfication and extraction of nuclear energy
... The name material point taken from classical mechanics refers to a particle of substance, not to a particle of matter which is a more general notion. Matter can be substance (the gravitational mass in mechanics) and radiation (the photon’s mass in electrodynamics). 60. The physical quantities. The p ...
... The name material point taken from classical mechanics refers to a particle of substance, not to a particle of matter which is a more general notion. Matter can be substance (the gravitational mass in mechanics) and radiation (the photon’s mass in electrodynamics). 60. The physical quantities. The p ...
Preliminary Course Atomic Structure 1 + 2
... (to do with the location of the electrons and the amount of energy they can have), but these will be dealt with in more detail later ...
... (to do with the location of the electrons and the amount of energy they can have), but these will be dealt with in more detail later ...
4 slides per page() - Wayne State University Physics and
... Elementary particle interactions The scattering of two electrons via a coulomb force This virtual photon is said to mediate the electromagnetic force. The virtual photon can never be detected because it only lasts for a vanishing small time. ...
... Elementary particle interactions The scattering of two electrons via a coulomb force This virtual photon is said to mediate the electromagnetic force. The virtual photon can never be detected because it only lasts for a vanishing small time. ...
Quantum Mechanics
... 4. Examine the pionic decays of K 0 governed by weak interactions. The dominant decays are K 0 → π + π − and K 0 → π 0 π 0 . Total isospin is not conserved in these processes, but changes either by 4I = + 12 or by 4I = − 12 . a. Introducing appropriate creation and annihilation operators, write dow ...
... 4. Examine the pionic decays of K 0 governed by weak interactions. The dominant decays are K 0 → π + π − and K 0 → π 0 π 0 . Total isospin is not conserved in these processes, but changes either by 4I = + 12 or by 4I = − 12 . a. Introducing appropriate creation and annihilation operators, write dow ...
Have a closer look at electron microscopy
... philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838;[3] Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identifie ...
... philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838;[3] Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identifie ...
Ideas On Containment of Physical Information Within the
... location of each gas particle has greatly decreased. The latter situation is a situation with high entropy; the former lower. If we were to measure the box at some later time and found all of the particle back in their original positions in the corner, then we would be VERY surprised. That particula ...
... location of each gas particle has greatly decreased. The latter situation is a situation with high entropy; the former lower. If we were to measure the box at some later time and found all of the particle back in their original positions in the corner, then we would be VERY surprised. That particula ...
Environmental Physics for Freshman Geography Students
... where q1 and q2 are the amounts of electric charge (measured in coulombs, C), r is the distance between them (measured in m), and K is Coulomb’s electrostatic constant (= 8.99 x 109 kg m3 s-2 C-2). The introduction of electric charges into the simple world of mechanics requires the use of a new dime ...
... where q1 and q2 are the amounts of electric charge (measured in coulombs, C), r is the distance between them (measured in m), and K is Coulomb’s electrostatic constant (= 8.99 x 109 kg m3 s-2 C-2). The introduction of electric charges into the simple world of mechanics requires the use of a new dime ...
Particles reactions - Teaching Advanced Physics
... Electrons colliding with positrons provide one of the most exciting ways of learning about bizarre varieties of matter…. The key feature is that positrons are the antiparticles of electrons. When matter and antimatter meet, they can mutually annihilate. The energy in their masses has been ...
... Electrons colliding with positrons provide one of the most exciting ways of learning about bizarre varieties of matter…. The key feature is that positrons are the antiparticles of electrons. When matter and antimatter meet, they can mutually annihilate. The energy in their masses has been ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.