Three-Body Problem
... The gravitational three-body problem is one of the oldest problems in mathematical physics. The goal is to determine the trajectories of three interacting particles. Historically, the system involving the Moon, the Earth and the Sun was the first three-body problem that received extended study. Sinc ...
... The gravitational three-body problem is one of the oldest problems in mathematical physics. The goal is to determine the trajectories of three interacting particles. Historically, the system involving the Moon, the Earth and the Sun was the first three-body problem that received extended study. Sinc ...
Atomic Theory Review
... The letters s, p, d, or f, are used to designate a particular __ within an energy level. ...
... The letters s, p, d, or f, are used to designate a particular __ within an energy level. ...
Attenuation of gamma particles
... coefficient, we have a physical interpretation for α. The linear attenuation coefficient is the probability per unit length that a gamma particle will undergo an interaction in the material. If α is large(small), the probability of an interaction is high(low) and the beam attenuates in a short(long) ...
... coefficient, we have a physical interpretation for α. The linear attenuation coefficient is the probability per unit length that a gamma particle will undergo an interaction in the material. If α is large(small), the probability of an interaction is high(low) and the beam attenuates in a short(long) ...
Introduction to Particle Physics
... • Worked on Large-Electron-Positron (LEP) and Large-Hadron-Collider(LHC) • Started as research associate professor @ the NBI Particle Physics group (HEP) this year • Office: Blegdamsvej 21 (building M) mc-8 • Email: [email protected] ...
... • Worked on Large-Electron-Positron (LEP) and Large-Hadron-Collider(LHC) • Started as research associate professor @ the NBI Particle Physics group (HEP) this year • Office: Blegdamsvej 21 (building M) mc-8 • Email: [email protected] ...
Everything You Wanted to Know About Quarks but were afraid to ask…
... Discovery of Nucleus Theory • Rutherford found that most collisions were at electron small angles but occasionally the a particles would bounce back. • “it was as if you fired 15” shells at tissue paper and they bounced back and hit you” Positive charge all inside small nucleus large angle scatte ...
... Discovery of Nucleus Theory • Rutherford found that most collisions were at electron small angles but occasionally the a particles would bounce back. • “it was as if you fired 15” shells at tissue paper and they bounced back and hit you” Positive charge all inside small nucleus large angle scatte ...
Types of Radiation
... Definition: A positively charged particle that consists of two protons and two neutrons bound together. It is emitted by an atomic nucleus undergoing radioactive decay and is identical to the nucleus of a helium atom. Because of their relatively large mass, alpha particles are the slowest and least ...
... Definition: A positively charged particle that consists of two protons and two neutrons bound together. It is emitted by an atomic nucleus undergoing radioactive decay and is identical to the nucleus of a helium atom. Because of their relatively large mass, alpha particles are the slowest and least ...
A little Big Bang
... gases of ultracold atoms, about one hundred million times colder than interstellar space and one hundred thousand times less dense than air, one can realize the phenomenon of Bose-Einstein condensation (BEC) in its purest form. In these ultradilute gases, over ninety-nine percent of the atoms, about ...
... gases of ultracold atoms, about one hundred million times colder than interstellar space and one hundred thousand times less dense than air, one can realize the phenomenon of Bose-Einstein condensation (BEC) in its purest form. In these ultradilute gases, over ninety-nine percent of the atoms, about ...
Press Release How atoms change places Physicists from the
... the spin up is initially located on the left side of the double well, it can be found on the right after a certain time, whereas the other atom with spin down which started from the right well will then be located on the left. The basis of this effect is a synchronous hopping event, or more formally ...
... the spin up is initially located on the left side of the double well, it can be found on the right after a certain time, whereas the other atom with spin down which started from the right well will then be located on the left. The basis of this effect is a synchronous hopping event, or more formally ...
worksheer format 11-12
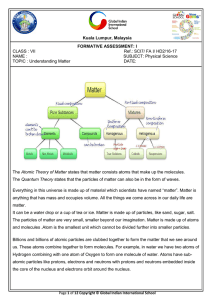
... It can be a water drop or a cup of tea or ice. Matter is made up of particles, like sand, sugar, salt. The particles of matter are very small, smaller beyond our imagination. Matter is made up of atoms and molecules .Atom is the smallest unit which cannot be divided further into smaller particles . ...
... It can be a water drop or a cup of tea or ice. Matter is made up of particles, like sand, sugar, salt. The particles of matter are very small, smaller beyond our imagination. Matter is made up of atoms and molecules .Atom is the smallest unit which cannot be divided further into smaller particles . ...
Lesson 18
... The center-of-mass potential energy is what we have been dealing with throughout this semester. We have neglected the energy which can be stored inside an object due to chemical, nuclear, and other interactions between the atoms and molecules which make up a real block, pulley, or other macroscopic ...
... The center-of-mass potential energy is what we have been dealing with throughout this semester. We have neglected the energy which can be stored inside an object due to chemical, nuclear, and other interactions between the atoms and molecules which make up a real block, pulley, or other macroscopic ...
Ch. 19: Electric charges, Forces, and Fields (Dr. Andrei Galiautdinov, UGA)
... Why divide Fe by qtest? – B/c it is found experimentally that, no matter how source charges move, the force Fe the test charge experiences at a given location is proportional to qtest itself. So, dividing by qtest produces a quantity E that depends only on position relative to the charges creating t ...
... Why divide Fe by qtest? – B/c it is found experimentally that, no matter how source charges move, the force Fe the test charge experiences at a given location is proportional to qtest itself. So, dividing by qtest produces a quantity E that depends only on position relative to the charges creating t ...
Chapter 13 - Gravitation
... The gravitational force is a conservative force. Thus, the work done by the gravitational force on a particle moving from an initial point i to a final point f is independent of the path taken between the points. The change DU in the gravitational potential energy from point i to point f is given by ...
... The gravitational force is a conservative force. Thus, the work done by the gravitational force on a particle moving from an initial point i to a final point f is independent of the path taken between the points. The change DU in the gravitational potential energy from point i to point f is given by ...
The Houghton College Cyclotron: Results and Modifications
... successfully on one occasion. Although no helium was introduced to the system, an RGA scan indicated there was sufficient residual hydrogen in the chamber to accelerate protons. The RF generator was set to 3.65 MHz, corresponding to a magnetic field strength of 0.24 T. A distinct peak in current col ...
... successfully on one occasion. Although no helium was introduced to the system, an RGA scan indicated there was sufficient residual hydrogen in the chamber to accelerate protons. The RF generator was set to 3.65 MHz, corresponding to a magnetic field strength of 0.24 T. A distinct peak in current col ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.