Specific Heat of Metals Make Up Directions
... 1. Go to the website listed above. Read the information and then scroll down to the applet. Uses JAVA so may need to update or allow JAVA to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record ...
... 1. Go to the website listed above. Read the information and then scroll down to the applet. Uses JAVA so may need to update or allow JAVA to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record ...
File
... A 9.84 oz ingot of unknown metal is heated from 73.2 °F to 191.2 °F. This requires 3.91 kcal of energy. Calculate the specific heat of the metal and determine its identity. ...
... A 9.84 oz ingot of unknown metal is heated from 73.2 °F to 191.2 °F. This requires 3.91 kcal of energy. Calculate the specific heat of the metal and determine its identity. ...
DriTherm®: Brick Cavity Wall Insulation
... Repels summer heat. Retains winter warmth. Between 15% and 25% of heat loss or gain in the home is through uninsulated cavity walls. If you are building your new home, now is the time to act to ensure it is comfortable all year round – cool in summer and warm in winter. ...
... Repels summer heat. Retains winter warmth. Between 15% and 25% of heat loss or gain in the home is through uninsulated cavity walls. If you are building your new home, now is the time to act to ensure it is comfortable all year round – cool in summer and warm in winter. ...
TEKNIK MESIN FAKULTAS TEKNOLOGI INDUSTRI UNIVERSITAS
... On a microscopic scale, thermal energy is related to the kinetic energy of molecules. The greater a material's temperature, the greater the thermal agitation of its constituent molecules (manifested both in linear motion and vibrational modes). It is natural for regions containing greater molecular ...
... On a microscopic scale, thermal energy is related to the kinetic energy of molecules. The greater a material's temperature, the greater the thermal agitation of its constituent molecules (manifested both in linear motion and vibrational modes). It is natural for regions containing greater molecular ...
Conductive Thermal Transfer
... coupled with an important contribution from the crust. So important questions include: • What is the transfer mechanism across the ductile lower crust? • Does the combination of “crustal” and “mantle” signatures reflect mixing, or residence time? • What are the flux rates? • How much heat was lost a ...
... coupled with an important contribution from the crust. So important questions include: • What is the transfer mechanism across the ductile lower crust? • Does the combination of “crustal” and “mantle” signatures reflect mixing, or residence time? • What are the flux rates? • How much heat was lost a ...
Process Heat Transfer Lab - University of Engineering and Technology
... e.g., petroleum processes involve a variety of heat exchangers and reactors whose performance is very much affected through heat transfer. The process heat transfer lab is designed to familiarize the students with the science of heat transfer so that they are able to apply the basic fundamentals for ...
... e.g., petroleum processes involve a variety of heat exchangers and reactors whose performance is very much affected through heat transfer. The process heat transfer lab is designed to familiarize the students with the science of heat transfer so that they are able to apply the basic fundamentals for ...
Thermodynamics lesson 1 Tempersture
... • oF is for old people, like pounds and ounces BUT conversion is a skill so lets not dispose of it all together • R just for some US engineers • oC not C. Centigrade just means that, we want Celsius, and degress at that. • K is not oK as it is absolute. small point but important ...
... • oF is for old people, like pounds and ounces BUT conversion is a skill so lets not dispose of it all together • R just for some US engineers • oC not C. Centigrade just means that, we want Celsius, and degress at that. • K is not oK as it is absolute. small point but important ...
Heat and the Umpire
... lacks the availability of shade, lacks the time that teams get to rest while they are up to bat, wears dark clothing, and minimal skin is exposed from which to allow heat to dissipate from your body. Add to this other issues such as hydration, cardiac issues, medical issues, medications and other fa ...
... lacks the availability of shade, lacks the time that teams get to rest while they are up to bat, wears dark clothing, and minimal skin is exposed from which to allow heat to dissipate from your body. Add to this other issues such as hydration, cardiac issues, medical issues, medications and other fa ...
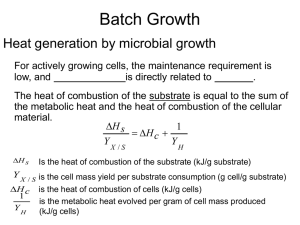
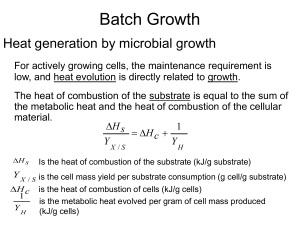
lecture notes-growth kinetics-3-heat evolution
... YX /S The higher degree of oxidation of the substrate has lower amounts of heat released: 1/YH ...
... YX /S The higher degree of oxidation of the substrate has lower amounts of heat released: 1/YH ...
Thermal Energy
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
heat evolution
... Y X / S is the cell mass yield per substrate consumption (g cell/g substrate) H c is the heat of combustion of cells (kJ/g cells) ...
... Y X / S is the cell mass yield per substrate consumption (g cell/g substrate) H c is the heat of combustion of cells (kJ/g cells) ...
Chemistry 2015-2016 Name: Calorimetry Practice Date: Per
... We don’t always have to use water. Let’s use some aluminum shot. 175 grams of hot aluminum (100.°C) is dropped into an insulated cup that contains 40.0 mL of ice cold water (0.0°C). Follow the example above to determine the final temperature, ...
... We don’t always have to use water. Let’s use some aluminum shot. 175 grams of hot aluminum (100.°C) is dropped into an insulated cup that contains 40.0 mL of ice cold water (0.0°C). Follow the example above to determine the final temperature, ...
Specific Heat Capacity
... Specific Heat Capacity Specific Heat Capacity (C or S ) - The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity of the substance. The quantity of heat is frequently measured in units of Joules(J). Another property, the specif ...
... Specific Heat Capacity Specific Heat Capacity (C or S ) - The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity of the substance. The quantity of heat is frequently measured in units of Joules(J). Another property, the specif ...
specific heat
... C. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat 2) low specific heat LecturePLUS Timberlake 99 ...
... C. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat 2) low specific heat LecturePLUS Timberlake 99 ...
Specific Heat WS #2 - My Chemistry Class
... How much heat is gained when a 50.32 g piece of aluminum is heated from 9.0°C to 16°C? ...
... How much heat is gained when a 50.32 g piece of aluminum is heated from 9.0°C to 16°C? ...
heat
... “Cold-blooded” animals, such as lizards and snakes, are exothermic as they rely on the sun to warm up. ...
... “Cold-blooded” animals, such as lizards and snakes, are exothermic as they rely on the sun to warm up. ...
Document
... plate and a 100 mm layer of insulation bricks. The maximum temperature of the wall is 11500C on the furnace side and the minimum temperature is 400C on the outermost side of the wall. An accurate energy balance over the furnace shows that the heat loss from the wall is 400 W/m2. It is known that the ...
... plate and a 100 mm layer of insulation bricks. The maximum temperature of the wall is 11500C on the furnace side and the minimum temperature is 400C on the outermost side of the wall. An accurate energy balance over the furnace shows that the heat loss from the wall is 400 W/m2. It is known that the ...
Heat Chapter 12: Thermodynamics
... • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Thermodynamics – It is not possible to lower temperatur ...
... • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Thermodynamics – It is not possible to lower temperatur ...
Heat Transfer (ME-345) - Department of Mechanical Engineering
... Heat Transfer (ME-345) EMU Mechanical Engineering Spring 2014-2015 Closed Book Quiz. Name: ...
... Heat Transfer (ME-345) EMU Mechanical Engineering Spring 2014-2015 Closed Book Quiz. Name: ...
First Law of Thermodynamics
... •It is a pressure •Pressure above the liquid=pressure from particles leaving the surface ...
... •It is a pressure •Pressure above the liquid=pressure from particles leaving the surface ...
Specific Heat and Calorimeters
... 9) A 5 g piece of copper metal is placed inside a cup of hot water. The copper penny, initially at 25 OC is heated to the temperature of the water, 49 OC. The amount of heat absorbed by the copper is 67.2 J. What is the specific heat of the copper metal? ...
... 9) A 5 g piece of copper metal is placed inside a cup of hot water. The copper penny, initially at 25 OC is heated to the temperature of the water, 49 OC. The amount of heat absorbed by the copper is 67.2 J. What is the specific heat of the copper metal? ...
Heat Calculations with Specific Heat
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...
... • EX. How much heat is necessary to totally melt 5 g of ice at 0 C to liquid water at 0 C? • EX. How much heat is necessary to change 5 g of water at 100 C to steam at 100 C? ...