Teacher`s notes 22 Specific Heat Capacity of a solid
... c = specific heat capacity The term specific in the physical sciences often refers to quantities divided by a specified reference quantity. When specific heat capacity is used, the term usually means mass-specific, or "per unit of mass." For example, water has a mass-specific heat capacity of about ...
... c = specific heat capacity The term specific in the physical sciences often refers to quantities divided by a specified reference quantity. When specific heat capacity is used, the term usually means mass-specific, or "per unit of mass." For example, water has a mass-specific heat capacity of about ...
Thermodynamics–Honors
... System = the area or space we focus on Surroundings = everything else apart from the system ...
... System = the area or space we focus on Surroundings = everything else apart from the system ...
Specific Heat Equation Practice Worksheet
... can be absorbed before the substance experiences a temperature change. This is called specific heat. Specific heat is the amount of energy that can be transferred as heat that will raise the temperature of 1 kg of a substance by 1°C. Imagine that you are at the pool on a hot summer day. Think of how ...
... can be absorbed before the substance experiences a temperature change. This is called specific heat. Specific heat is the amount of energy that can be transferred as heat that will raise the temperature of 1 kg of a substance by 1°C. Imagine that you are at the pool on a hot summer day. Think of how ...
Advantages of Plate-Type Heat Exchanger over Tube-Type
... Advantages of Plate-Type Heat Exchanger over Tube-Type Heat Exchanger for OTEC Power Plant Tomohiro Mitsumori, Yasuyuki Ikegami and Haruo Uehara OTEC Laboratory, Saga University, Saga, Japan INTRODUCTION A high-performance heat exchanger should be used in the evaporator and condenser of Ocean Therma ...
... Advantages of Plate-Type Heat Exchanger over Tube-Type Heat Exchanger for OTEC Power Plant Tomohiro Mitsumori, Yasuyuki Ikegami and Haruo Uehara OTEC Laboratory, Saga University, Saga, Japan INTRODUCTION A high-performance heat exchanger should be used in the evaporator and condenser of Ocean Therma ...
2nd law - WordPress.com
... mechanical, chemical and thermal equilibrium are not satisfied, and the dissipative effects are present. For a process to be reversible, it must not posses these features. If a process is performed quasi-statically, the system passes through states of thermodynamic equilibrium, which may be travers ...
... mechanical, chemical and thermal equilibrium are not satisfied, and the dissipative effects are present. For a process to be reversible, it must not posses these features. If a process is performed quasi-statically, the system passes through states of thermodynamic equilibrium, which may be travers ...
BCJ0205-15 Thermal phenomena (3-1-4)
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
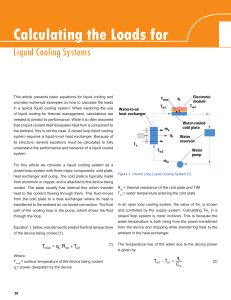
Calculating the Loads for Liquid cooling Systems
... This article presents basic equations for liquid cooling and provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed th ...
... This article presents basic equations for liquid cooling and provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed th ...
Heat Transfer and Energy
... which is why styrofoam cups are used to hold hot coffee. • In general, metals have very large conductivities. Thus, when they are heated, molecules will rapidly transfer heat through the metal. ...
... which is why styrofoam cups are used to hold hot coffee. • In general, metals have very large conductivities. Thus, when they are heated, molecules will rapidly transfer heat through the metal. ...
Too Hot to Handle, Too Cold to Hold
... 1) For “high” temperature applications, gas saves 67% on energy and cost. (Hotter than you want to touch). 2) For space/water heating, we have an excess of waste heat from power plants and an abundance of geothermal heat. 3) To fill in gaps and further reduce costs, integrated renewables (wind/solar ...
... 1) For “high” temperature applications, gas saves 67% on energy and cost. (Hotter than you want to touch). 2) For space/water heating, we have an excess of waste heat from power plants and an abundance of geothermal heat. 3) To fill in gaps and further reduce costs, integrated renewables (wind/solar ...
File - Ms. A Science Online
... popsicle. The room is warmer than the popsicle. So heat energy travels from the room to the popsicle, first melting it and then warming the liquid to room temperature. Heat energy always moves from HOT to COLD ! ...
... popsicle. The room is warmer than the popsicle. So heat energy travels from the room to the popsicle, first melting it and then warming the liquid to room temperature. Heat energy always moves from HOT to COLD ! ...
Heat on the move
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
... Heat can move from one place to another – heat Put the three spoons into the container of hot is transferred. It always moves from somewhere water. Feel the ends of the spoons. Which of the or something hot to a place or object that’s cooler. ends became hot? Can you think of some examples? When hea ...
Transferts couplés de masse et de chaleur dans une mousse solide
... knowledge of all mechanisms which govern heat and mass transfers during various processes: baking, chilling and eventually freezing and thawing. This paper presents a synthesis of works achieved to fill this gap. The objective is to develop a coupled heat and mass transfers model in the case of soli ...
... knowledge of all mechanisms which govern heat and mass transfers during various processes: baking, chilling and eventually freezing and thawing. This paper presents a synthesis of works achieved to fill this gap. The objective is to develop a coupled heat and mass transfers model in the case of soli ...
Thermodynamics Guided Notes
... This packet will contain a wealth of knowledge on the topics to be covered in chapters 2124. The information will be covered in 3 ways. First, I will assign you sections to read from your Conceptual Physics book. Then we will take guided notes in class over the material from the sections you preciou ...
... This packet will contain a wealth of knowledge on the topics to be covered in chapters 2124. The information will be covered in 3 ways. First, I will assign you sections to read from your Conceptual Physics book. Then we will take guided notes in class over the material from the sections you preciou ...
Lab 1
... container and measure the temperature of the warm water. If you start with water about 5 oC to 10oC above room temperature and end with the water about 5 oC to 10oC below room temperature, the heat that sneaks into the cooler room from the warm water will nearly cancel the heat that sneaks into the ...
... container and measure the temperature of the warm water. If you start with water about 5 oC to 10oC above room temperature and end with the water about 5 oC to 10oC below room temperature, the heat that sneaks into the cooler room from the warm water will nearly cancel the heat that sneaks into the ...
Specific Heat
... Learning Check 2. Two objects are sitting next to each other in the sunlight. Object A gets hotter than object B. A. Object A has a lower specific heat than object B B. Object A has a higher specific heat than object B C. Both objects have the same specific heat ...
... Learning Check 2. Two objects are sitting next to each other in the sunlight. Object A gets hotter than object B. A. Object A has a lower specific heat than object B B. Object A has a higher specific heat than object B C. Both objects have the same specific heat ...
EML 6154 - UFL MAE - University of Florida
... Objectives: The goal of this course is to teach basic and advanced solution techniques, including exact and approximate approaches, for a wide range of conduction heat transfer problems. Included are both multidimensional steady state and transient analyses, with emphasis on the fundamental physics ...
... Objectives: The goal of this course is to teach basic and advanced solution techniques, including exact and approximate approaches, for a wide range of conduction heat transfer problems. Included are both multidimensional steady state and transient analyses, with emphasis on the fundamental physics ...
5.2 Solid Matter
... • The word amorphous comes from the Greek for “without shape.” • Unlike crystalline solids, amorphous solids do not have a repeating pattern of molecules or atoms. ...
... • The word amorphous comes from the Greek for “without shape.” • Unlike crystalline solids, amorphous solids do not have a repeating pattern of molecules or atoms. ...
5.2 Solid Matter
... • The word amorphous comes from the Greek for “without shape.” • Unlike crystalline solids, amorphous solids do not have a repeating pattern of molecules or atoms. ...
... • The word amorphous comes from the Greek for “without shape.” • Unlike crystalline solids, amorphous solids do not have a repeating pattern of molecules or atoms. ...
thermodynamics
... silver bullets! Anyway she spots one of the fearsome beasts and fires off a round, but misses! The bullet drills into a thick slab of insulating material. If the bullet has a mass of 3.50 g and a speed of 225 m/s, what is its final temperature when it comes to rest (csilver = 0.23 J/gAK)? Assuming a ...
... silver bullets! Anyway she spots one of the fearsome beasts and fires off a round, but misses! The bullet drills into a thick slab of insulating material. If the bullet has a mass of 3.50 g and a speed of 225 m/s, what is its final temperature when it comes to rest (csilver = 0.23 J/gAK)? Assuming a ...
Document
... Pour a liter of water at 40 degrees C into a liter of water at 20 degrees C and the final temperature of the two becomes A) less than 30 degrees C. B) at or about 30 degrees C. C) more than 30 degrees C. ...
... Pour a liter of water at 40 degrees C into a liter of water at 20 degrees C and the final temperature of the two becomes A) less than 30 degrees C. B) at or about 30 degrees C. C) more than 30 degrees C. ...
... f) Find the net work produced by the air during one cycle. An engine working with 0.1 kg of air follows the Camot cycle. The high temperature reservoir is at 940 K, and at the beginning of isothermal expansion at this temperature the air pressure is 8.4 MPa. The heat added to the air in this expansi ...
Werribee Centrals FNC in conjunction with AFL AND
... • during exercise longer than 60 minutes, 2-3 cups (500-700ml) of cool water or sports drink are sufficient for most sports. • after exercise replenish your fluid deficit to ensure that you are fully re- hydrated, but not overhydrated. ...
... • during exercise longer than 60 minutes, 2-3 cups (500-700ml) of cool water or sports drink are sufficient for most sports. • after exercise replenish your fluid deficit to ensure that you are fully re- hydrated, but not overhydrated. ...