have shown no evidence
... and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) until by element 83 (Bi, the last one with a stable is ...
... and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) until by element 83 (Bi, the last one with a stable is ...
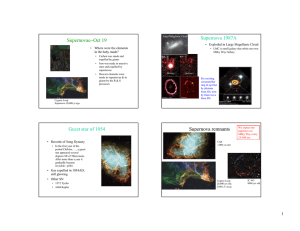
Supernovae Oct 19 − Supernova 1987A
... What is the only element at the start? How many neutrons does it have? At what time did some gold form? Gold has 79 protons. Is this gold stable? At the end of the calculation, how many protons does the nucleus with the most protons have? What is the time at the end of the calculation? Are the end p ...
... What is the only element at the start? How many neutrons does it have? At what time did some gold form? Gold has 79 protons. Is this gold stable? At the end of the calculation, how many protons does the nucleus with the most protons have? What is the time at the end of the calculation? Are the end p ...
Nuclear Chemistry Test Topics
... Radiation consists of particles and energy being given off by unstable nuclei. It cannot be seen or felt, but it is harmful to living things. An isotope of an element differs in the number of neutrons it contains. To determine the mass number of an atom, add the number of protons and the number of n ...
... Radiation consists of particles and energy being given off by unstable nuclei. It cannot be seen or felt, but it is harmful to living things. An isotope of an element differs in the number of neutrons it contains. To determine the mass number of an atom, add the number of protons and the number of n ...
Stellar Evolution
... Ring due to SN ejecta catching up with preSN stellar wind; also observable in X-rays. ...
... Ring due to SN ejecta catching up with preSN stellar wind; also observable in X-rays. ...
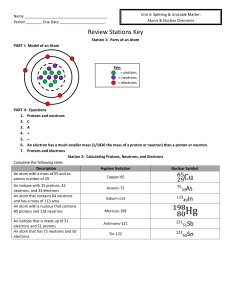
Name Period ______ Due Date Review Stations Key Station 1
... atomic number of 29 An isotope with 33 protons, 42 neutrons, and 33 electrons An atom that contains 64 neutrons and has a mass of 113 amu An atom with a nucleus that contains 80 protons and 118 neutrons An isotope that is made up of 51 electrons and 51 protons An atom that has 72 neutrons and 50 ele ...
... atomic number of 29 An isotope with 33 protons, 42 neutrons, and 33 electrons An atom that contains 64 neutrons and has a mass of 113 amu An atom with a nucleus that contains 80 protons and 118 neutrons An isotope that is made up of 51 electrons and 51 protons An atom that has 72 neutrons and 50 ele ...
Lesson 13 - Oregon State University
... • Population II stars (H, He, 1% heavier elements) • Population I stars (H, He, 2-5% heavier elements) Includes our sun. ...
... • Population II stars (H, He, 1% heavier elements) • Population I stars (H, He, 2-5% heavier elements) Includes our sun. ...
Chemistry Standard 2A-Nucleus Section 20.1
... c. number of neutrons b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is found ____________ a. radioactivity c. decomposition b. oxidation d. none of the above 6. When the ____ is not large ...
... c. number of neutrons b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is found ____________ a. radioactivity c. decomposition b. oxidation d. none of the above 6. When the ____ is not large ...
L37 - University of Iowa Physics
... other living thing, but the carbon-14 decays and is not replaced. The carbon-14 decays with its halflife of 5,700 years, while the amount of carbon-12 remains constant in the sample. By looking at the ratio of carbon-12 to carbon-14 in the sample and comparing it to the ratio in a living organism, i ...
... other living thing, but the carbon-14 decays and is not replaced. The carbon-14 decays with its halflife of 5,700 years, while the amount of carbon-12 remains constant in the sample. By looking at the ratio of carbon-12 to carbon-14 in the sample and comparing it to the ratio in a living organism, i ...
Radioactivity - Teach Nuclear
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
Nuclear Exotica (online version) - ECM-UB
... production of exotic nuclei with very different N, Z numbers from the known isotopes achieved with RIB facilities experiments start unveiling the properties of short lived, weakly bound nuclei far from stability extend our knowledge of nuclear structure Major next generation facilities planned o ...
... production of exotic nuclei with very different N, Z numbers from the known isotopes achieved with RIB facilities experiments start unveiling the properties of short lived, weakly bound nuclei far from stability extend our knowledge of nuclear structure Major next generation facilities planned o ...
29 October: Dead Stars 3
... Note in particular 40 Eridani B, which is in The sky now, and can be seen with small telescopes ...
... Note in particular 40 Eridani B, which is in The sky now, and can be seen with small telescopes ...
Unit 14 Notes - shscience.net
... electron but a positive charge During positron emission, a proton changes to a neutron and positron (which is emitted) Atomic # , mass # doesn’t change ...
... electron but a positive charge During positron emission, a proton changes to a neutron and positron (which is emitted) Atomic # , mass # doesn’t change ...
Nuclear Chemistry
... nuclear forces that overcome the electromagnetic repulsion between the protons. b. Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc2) is small but significant in nuclear re ...
... nuclear forces that overcome the electromagnetic repulsion between the protons. b. Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc2) is small but significant in nuclear re ...
Radioactive Decay
... Atoms are likely to undergo β+ decay if they have too many protons and not enough neutrons to achieve a stable neutron/proton ratio. Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay, but β+ decay is always accomp ...
... Atoms are likely to undergo β+ decay if they have too many protons and not enough neutrons to achieve a stable neutron/proton ratio. Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay, but β+ decay is always accomp ...
stellar_explosions - UT Austin (Astronomy)
... Death of a High-Mass Star In short: Envelope explodes as a core collapse supernova. The core implodes and ends up as a neutron star or (more massive) a black hole. Let’s see how this occurs. (Remember, this is all theoretical calculations, but later you’ll see that there is surprising observational ...
... Death of a High-Mass Star In short: Envelope explodes as a core collapse supernova. The core implodes and ends up as a neutron star or (more massive) a black hole. Let’s see how this occurs. (Remember, this is all theoretical calculations, but later you’ll see that there is surprising observational ...
nucleosynthesis_oct28
... Stellar Nucleosynthesis, t = 400 Myr to 13.7 Gyr According to the Wilkinson Microwave Anisotropy Probe (WMAP) the first stars, called population III stars, started shining at t = 400 Myr. These stars started their lives with the primordial nuclear composition. The initial mass distribution of thes ...
... Stellar Nucleosynthesis, t = 400 Myr to 13.7 Gyr According to the Wilkinson Microwave Anisotropy Probe (WMAP) the first stars, called population III stars, started shining at t = 400 Myr. These stars started their lives with the primordial nuclear composition. The initial mass distribution of thes ...
Proton spectra
... Proton chemical shifts generally run from 0 to 12 ppm and are referenced from tetramethylsilane (TMS, (CH3)4Si). The lower chemical shifts are associated with protons that have a high electron density and are in what is known as the high field region. ...
... Proton chemical shifts generally run from 0 to 12 ppm and are referenced from tetramethylsilane (TMS, (CH3)4Si). The lower chemical shifts are associated with protons that have a high electron density and are in what is known as the high field region. ...
Life Cycle of Stars: Chapter 21
... Life as High-Mass Star = 8x’s sun • Birthed from nebula • Core contains convective zone • Nuclear fusion of heavier elements – Requires high temperatures – More gravitational contraction ...
... Life as High-Mass Star = 8x’s sun • Birthed from nebula • Core contains convective zone • Nuclear fusion of heavier elements – Requires high temperatures – More gravitational contraction ...
What stars do Summary: Nuclear burning in stars •
... blowing away outer 25% of their mass because of intense energy production in middle layers of star. ...
... blowing away outer 25% of their mass because of intense energy production in middle layers of star. ...
a Supernova!
... releases gravitational potential energy causes extreme heating (but not enough to halt the collapse and support the star) resulting in lots of very energetic radiation (gamma rays). ...
... releases gravitational potential energy causes extreme heating (but not enough to halt the collapse and support the star) resulting in lots of very energetic radiation (gamma rays). ...
P-nuclei
p-Nuclei (p stands for proton-rich) are certain proton-rich, naturally occurring isotopes of some elements between selenium and mercury which cannot be produced in either s- or r-process.