Sodasorb Manual - Shearwater Research
... Atlantic for their euphoric and exhilarating effects as a popular social pastime, casually referred to by many as a frolic. Still, it was some time before these agents were put to use in anesthesia. Surgeons continued to operate on patients who were either drunk, tied down, or both. Americans were t ...
... Atlantic for their euphoric and exhilarating effects as a popular social pastime, casually referred to by many as a frolic. Still, it was some time before these agents were put to use in anesthesia. Surgeons continued to operate on patients who were either drunk, tied down, or both. Americans were t ...
Removal of Chlorine Removal of Chlorine
... Zr(OH)4 comprised of bridging and terminal –OH groups Peak at 531.6 eV assigned to terminal hydroxyl groups Peak at 529.9 eV assigned to bridging hydroxyl group Note decrease in intensity of terminal –OH following chemical exposure In case of HCl exposed media, virtually no terminal –OH groups detec ...
... Zr(OH)4 comprised of bridging and terminal –OH groups Peak at 531.6 eV assigned to terminal hydroxyl groups Peak at 529.9 eV assigned to bridging hydroxyl group Note decrease in intensity of terminal –OH following chemical exposure In case of HCl exposed media, virtually no terminal –OH groups detec ...
3.0 Properties of Phosgene
... take place in the piping prior to the reactors. Normally, these impurities are at very low concentrations and the impurities formed are not significant. If a high concentration of either impurity exists, these reactions can generate enough heat to melt the pipe. Since chlorine is an oxidizer, and me ...
... take place in the piping prior to the reactors. Normally, these impurities are at very low concentrations and the impurities formed are not significant. If a high concentration of either impurity exists, these reactions can generate enough heat to melt the pipe. Since chlorine is an oxidizer, and me ...
C1a Revision notes - Calthorpe Park Moodle
... Distillation is a process that can be used to separate a pure liquid from a mixture of liquids. It works when the liquids have different boiling points. Distillation is commonly used to separate ethanol (the alcohol in alcoholic drinks) from water. The mixture is heated in a flask. Ethanol has a low ...
... Distillation is a process that can be used to separate a pure liquid from a mixture of liquids. It works when the liquids have different boiling points. Distillation is commonly used to separate ethanol (the alcohol in alcoholic drinks) from water. The mixture is heated in a flask. Ethanol has a low ...
T640, T642, T644 High Yield Return Program Print
... Like many finely divided materials, toner dust, in high concentrations can form an explosive mixture in air which, if ignited, could result in a dust explosion. ...
... Like many finely divided materials, toner dust, in high concentrations can form an explosive mixture in air which, if ignited, could result in a dust explosion. ...
BURNERS AND FLAMES:
... Sunlight can be split by water droplets into the spectrum of light called a rainbow. This same phenomenon can be observed when a beam of white light is passed through a prism. These observations led early scientists to the conclusion that white light was actually made up of all colors and that under ...
... Sunlight can be split by water droplets into the spectrum of light called a rainbow. This same phenomenon can be observed when a beam of white light is passed through a prism. These observations led early scientists to the conclusion that white light was actually made up of all colors and that under ...
13.IVA group. Carbon and Silicon and their compounds.
... luster. Silicon is rather strong, very brittle, and prone to chipping. Silicon, like carbon and germanium, crystallizes in a diamond cubic crystal structure. ...
... luster. Silicon is rather strong, very brittle, and prone to chipping. Silicon, like carbon and germanium, crystallizes in a diamond cubic crystal structure. ...
How many grams of oxygen are made if 3.75 moles of KClO 3
... to produce sodium chloride. How many grams of salt will be produced if 13.3 X 102 grams of sodium metal is available? ...
... to produce sodium chloride. How many grams of salt will be produced if 13.3 X 102 grams of sodium metal is available? ...
Gas Chromatography
... proteins, nucleotides, and pharmaceuticals can be studied with flame ionization as well as other detectors, like thermal conductivity, thermionic, or electrolytic conductivity due to the presence of nitrogen, phosphorus, or sulfur atoms or because of the universality of the thermal conductivity dete ...
... proteins, nucleotides, and pharmaceuticals can be studied with flame ionization as well as other detectors, like thermal conductivity, thermionic, or electrolytic conductivity due to the presence of nitrogen, phosphorus, or sulfur atoms or because of the universality of the thermal conductivity dete ...
material safety data sheet - Jon-Don
... Communication Standard (29 CFR 1910.1200) and the Hazardous Products Act (Can.) and the Controlled Products Regulations (Can.) This product has been classified in accordance with the hazard criteria of the CPR (Can.) and the MSDS contains all the information required by the CPR (Can.). This product ...
... Communication Standard (29 CFR 1910.1200) and the Hazardous Products Act (Can.) and the Controlled Products Regulations (Can.) This product has been classified in accordance with the hazard criteria of the CPR (Can.) and the MSDS contains all the information required by the CPR (Can.). This product ...
formula - eduBuzz.org
... • Can you draw a diagram of the experiment you would use to prove the products of combustion of carbon or a hydrocarbon? • What is the word and formula equation for carbon burning in air? • What is carbon dioxide used for? ...
... • Can you draw a diagram of the experiment you would use to prove the products of combustion of carbon or a hydrocarbon? • What is the word and formula equation for carbon burning in air? • What is carbon dioxide used for? ...
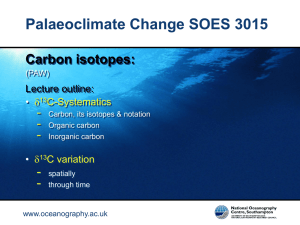
- EdShare - University of Southampton
... This resource was created by the University of Southampton and released as an open educational resource through the 'Cchange in GEES' project exploring the open licensing of climate change and sustainability resources in the Geography, Earth and Environmental Sciences. The C-change in GEES project w ...
... This resource was created by the University of Southampton and released as an open educational resource through the 'Cchange in GEES' project exploring the open licensing of climate change and sustainability resources in the Geography, Earth and Environmental Sciences. The C-change in GEES project w ...
qp13 - Smart Edu Hub
... 27 Fertilisers need to supply crops with three main elements. Which compound contains all three of these elements? A ...
... 27 Fertilisers need to supply crops with three main elements. Which compound contains all three of these elements? A ...
Section 7.1 Describing Reactions
... headings, vocabulary, and figures in this section. List two things you expect to learn. After reading, state what you learned about each item you listed. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. ...
... headings, vocabulary, and figures in this section. List two things you expect to learn. After reading, state what you learned about each item you listed. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. ...
Practice Problems
... maximum amount of magnesium oxide that could form? In other words, calculate the theoretical yield of MgO. FIRST: calculate the limiting reagent by finding the maximum amount of product (MgO) that could form IF each reactant was fully consumed. THEN: compare the amount of product that could form in ...
... maximum amount of magnesium oxide that could form? In other words, calculate the theoretical yield of MgO. FIRST: calculate the limiting reagent by finding the maximum amount of product (MgO) that could form IF each reactant was fully consumed. THEN: compare the amount of product that could form in ...
General Chemistry I
... How many grams of magnesium oxide will result when 10.0 g of magnesium ribbon is burned in air? 2Mg (s) + O2 (s) ...
... How many grams of magnesium oxide will result when 10.0 g of magnesium ribbon is burned in air? 2Mg (s) + O2 (s) ...
E/F Physical Science
... 1. Is the following sentence true or false? The new substances formed as a result of a chemical reaction are called products. 2. Circle the letter of each sentence that is correct for the chemical equation: C + O2 → CO2. a. Carbon and oxygen react and form carbon monoxide. b. Carbon and oxygen react ...
... 1. Is the following sentence true or false? The new substances formed as a result of a chemical reaction are called products. 2. Circle the letter of each sentence that is correct for the chemical equation: C + O2 → CO2. a. Carbon and oxygen react and form carbon monoxide. b. Carbon and oxygen react ...
53 word equations
... the reactants come in front of the arrow the products come after the arrow ...
... the reactants come in front of the arrow the products come after the arrow ...
File
... different chemical compounds – it is not much use straight out of the ground (there are too many substances in it, all with different boiling points). As such it needs to be refined… ...
... different chemical compounds – it is not much use straight out of the ground (there are too many substances in it, all with different boiling points). As such it needs to be refined… ...
zzz Sept 28 day thirteen
... parts of earth’s four spheres (eg. much CO2 is dissolved in the oceans, carbon is stored in the earths crust as coal and fossil fuels) ...
... parts of earth’s four spheres (eg. much CO2 is dissolved in the oceans, carbon is stored in the earths crust as coal and fossil fuels) ...
Carbon monoxide detector
A carbon monoxide detector or CO detector is a device that detects the presence of the carbon monoxide (CO) gas in order to prevent carbon monoxide poisoning. In the late 1990s Underwriters Laboratories (UL) changed their definition of a single station CO detector with a sound device in it to a carbon monoxide (CO) alarm. This applies to all CO safety alarms that meet UL 2034; however for passive indicators and system devices that meet UL 2075, UL refers to these as carbon monoxide detectors. CO is a colorless, tasteless and odorless compound produced by incomplete combustion of carbon containing materials. It is often referred to as the ""silent killer"" because it is virtually undetectable without using detection technology and most do not realise they are being poisoned. Elevated levels of CO can be dangerous to humans depending on the amount present and length of exposure. Smaller concentrations can be harmful over longer periods of time while increasing concentrations require diminishing exposure times to be harmful.CO detectors are designed to measure CO levels over time and sound an alarm before dangerous levels of CO accumulate in an environment, giving people adequate warning to safely ventilate the area or evacuate. Some system-connected detectors also alert a monitoring service that can dispatch emergency services if necessary.While CO detectors do not serve as smoke detectors and vice versa, dual smoke/CO detectors are also sold. Smoke detectors detect the smoke generated by flaming or smoldering fires, whereas CO detectors detect and warn people about dangerous CO buildup caused, for example, by a malfunctioning fuel-burning device. In the home, some common sources of CO include open flames, space heaters, water heaters, blocked chimneys or running a car inside a garage.