Question paper
... C N s2 kg–1 D N2 s2 (Total for Question 2 = 1 mark) 3 A radium nucleus decays by emitting an alpha particle. The speed of the recoiling nucleus is small compared to the speed of the alpha particle. This is because the A force acting on the recoiling nucleus is smaller than the force acting on the al ...
... C N s2 kg–1 D N2 s2 (Total for Question 2 = 1 mark) 3 A radium nucleus decays by emitting an alpha particle. The speed of the recoiling nucleus is small compared to the speed of the alpha particle. This is because the A force acting on the recoiling nucleus is smaller than the force acting on the al ...
Conduction and Semiconductors
... Electron band diagrams are a way to visualize what happens at a p-n junction, using the following rules: 1. The Fermi level must be at the same level on both sides of the junction when there is no applied field 2. Far from the junctions, the materials inherent electrical structure exists 3. He band ...
... Electron band diagrams are a way to visualize what happens at a p-n junction, using the following rules: 1. The Fermi level must be at the same level on both sides of the junction when there is no applied field 2. Far from the junctions, the materials inherent electrical structure exists 3. He band ...
30-32: Main Topics
... quantum mechanical picture • involves 4 quantum numbers (3 more than the Bohr model) • quantum-mechanical model allows for electron states with zero angular momentum ...
... quantum mechanical picture • involves 4 quantum numbers (3 more than the Bohr model) • quantum-mechanical model allows for electron states with zero angular momentum ...
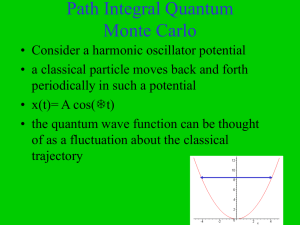
Path Integral Quantum Monte Carlo
... • L is the Lagrangian L=T-V • similarly, quantum mechanics can be formulated in terms of the Schrodinger equation or in terms of the action • the real time propagator can be expresssed as ...
... • L is the Lagrangian L=T-V • similarly, quantum mechanics can be formulated in terms of the Schrodinger equation or in terms of the action • the real time propagator can be expresssed as ...
The Atom
... you can know both the position and velocity of an object with perfect accuracy if given the mass C you can know both the position and velocity of an object with perfect accuracy if given an accurate value for Planck’s constant ...
... you can know both the position and velocity of an object with perfect accuracy if given the mass C you can know both the position and velocity of an object with perfect accuracy if given an accurate value for Planck’s constant ...
CMock exam IV paper 2
... 1M for starting from (all ice cubes have melted), 1A for approaching room temperature 2. (a) Temperature (of gas) 1M Mass of air/gas Or number of atoms/molecules/moles of air/gas 1M (b) Assumption: idea that volume occupied by trapped air proportional to length of air in tube (i.e. volume = cross-se ...
... 1M for starting from (all ice cubes have melted), 1A for approaching room temperature 2. (a) Temperature (of gas) 1M Mass of air/gas Or number of atoms/molecules/moles of air/gas 1M (b) Assumption: idea that volume occupied by trapped air proportional to length of air in tube (i.e. volume = cross-se ...
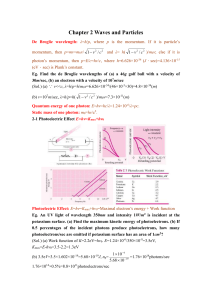
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... The shortest wavelength of X-ray due to electron bombard: λmin=1.24×10-6/V, where V is the accelerating voltage. Eg. Find the shortest wavelength present in the radiation from an X-ray machine whose accelerating potential is 50000V. (Sol.) λmin=1.24×10-6/50000=2.5×10-11m=0.025nm 2-2 Bragg’s Reflecti ...
... The shortest wavelength of X-ray due to electron bombard: λmin=1.24×10-6/V, where V is the accelerating voltage. Eg. Find the shortest wavelength present in the radiation from an X-ray machine whose accelerating potential is 50000V. (Sol.) λmin=1.24×10-6/50000=2.5×10-11m=0.025nm 2-2 Bragg’s Reflecti ...
ELECTROMAGNETIC ANALOGUE OF A POINT STRUCTURAL
... Since the early studies of Schrödinger [1] on building a classical theory of a particle with spin, a lot of work has been carried out in order to develop models and to analyze physical implications of such particle. The main difficulty of the large number of theoretical studies devoted to this matte ...
... Since the early studies of Schrödinger [1] on building a classical theory of a particle with spin, a lot of work has been carried out in order to develop models and to analyze physical implications of such particle. The main difficulty of the large number of theoretical studies devoted to this matte ...
Examination Paper (Mechanics)
... (6) A rigid body is made of three identical thin rods, each with length L, fastened together in the form of a letter H, as shown in the diagram. The body is free to rotate about a horizontal axis that runs along the length of one of the legs of the H. The body is allowed to fall from rest from a pos ...
... (6) A rigid body is made of three identical thin rods, each with length L, fastened together in the form of a letter H, as shown in the diagram. The body is free to rotate about a horizontal axis that runs along the length of one of the legs of the H. The body is allowed to fall from rest from a pos ...
Lecture 14
... ω = 10.2 eV × 1.6 × 10-19 J/eV / 1.05×10-43 J-s = 1.55 × 10 16 / s f = ω / 2 π = 2.47 × 1015 Hz and λ = c / f = (3 × 108 m/s) / (2.47 × 1015 /s) = 1.2 × 10-7 m, which is much larger than 10-10 m, which is the atomic diameter. In general, electronic transitions in atoms give light that is about 1 μm ...
... ω = 10.2 eV × 1.6 × 10-19 J/eV / 1.05×10-43 J-s = 1.55 × 10 16 / s f = ω / 2 π = 2.47 × 1015 Hz and λ = c / f = (3 × 108 m/s) / (2.47 × 1015 /s) = 1.2 × 10-7 m, which is much larger than 10-10 m, which is the atomic diameter. In general, electronic transitions in atoms give light that is about 1 μm ...
Honors Chemistry
... 10. What is meant by an electron having dual wave-particle nature, where were these electrons described as being located, and who suggested this theory? Sometimes light acts like a wave and some other times like a particle. To understand what light is one must take both characteristics into consider ...
... 10. What is meant by an electron having dual wave-particle nature, where were these electrons described as being located, and who suggested this theory? Sometimes light acts like a wave and some other times like a particle. To understand what light is one must take both characteristics into consider ...
quantum - Academia Sinica
... 1914, Bohr became famous after the success of his atomic model, and the Royal Danish Academy of Science gave him financial support to set up an Physics Institute. The fund was actually donated by Carlsberg ...
... 1914, Bohr became famous after the success of his atomic model, and the Royal Danish Academy of Science gave him financial support to set up an Physics Institute. The fund was actually donated by Carlsberg ...
Intro to Chapter 5 Development of the Periodic Table
... What properties of atoms is responsible for the periodic variations? To understand how, it s necessary to look first at the nature of visible line and other forms of radiant energy. Historically, studies of the interaction of radiant energy with matter provided immense insight into the atomic struct ...
... What properties of atoms is responsible for the periodic variations? To understand how, it s necessary to look first at the nature of visible line and other forms of radiant energy. Historically, studies of the interaction of radiant energy with matter provided immense insight into the atomic struct ...