What is matter made of?
... the case of atoms, scientists use large models to explain something that is very very small (invisible to the human eye) ...
... the case of atoms, scientists use large models to explain something that is very very small (invisible to the human eye) ...
THE ATOM
... B. In 1912, Max von Laue showed that X-rays are extremely high frequency em waves. C. X-rays are produced by high energy electrons that are stopped suddenly; the electron KE is transformed into photon energy. ...
... B. In 1912, Max von Laue showed that X-rays are extremely high frequency em waves. C. X-rays are produced by high energy electrons that are stopped suddenly; the electron KE is transformed into photon energy. ...
PHYS1111
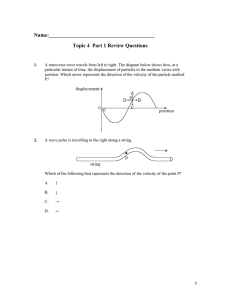
... What is the total energy of the system? c) What is the kinetic energy of the system as it passes through the equilibrium position? d) Where is the mass 1.5s after it is released? ...
... What is the total energy of the system? c) What is the kinetic energy of the system as it passes through the equilibrium position? d) Where is the mass 1.5s after it is released? ...
Physical Chemistry (4): Theoretical Chemistry
... where A depends on the nature of a metal plate (called work function). Explanation was given by Einstein again, using the quantization introduced by Planck: the light consist of tiny particles which can have energy of hν only (photon). (Note that Planck opposed the use of his „uncompleted” theory!!) ...
... where A depends on the nature of a metal plate (called work function). Explanation was given by Einstein again, using the quantization introduced by Planck: the light consist of tiny particles which can have energy of hν only (photon). (Note that Planck opposed the use of his „uncompleted” theory!!) ...
Chapter 5 Practice Section 5-1 Discuss the placement (if any) of
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
Honors Chemistry
... Students should be able to identify these elements simply based on total number of electrons Significance of electron configurations Valence shell electrons - outermost electrons involved with bonding for n = 5, pattern is very complicated - no atom has more than 8 valence electrons Noble gases - 8 ...
... Students should be able to identify these elements simply based on total number of electrons Significance of electron configurations Valence shell electrons - outermost electrons involved with bonding for n = 5, pattern is very complicated - no atom has more than 8 valence electrons Noble gases - 8 ...
Langevin Equation
... To understand the Brownian motion more completely, we need to start from the basic physics, i.e. Newton’s law of motion. The most direct way of implementing this is to recognize that there is a stochastic component to the force on the particle, which we only know through a probabilistic description. ...
... To understand the Brownian motion more completely, we need to start from the basic physics, i.e. Newton’s law of motion. The most direct way of implementing this is to recognize that there is a stochastic component to the force on the particle, which we only know through a probabilistic description. ...
Lecture 18 (Slides) October 4
... • We will use Slide 12 to determine when calculus must be used to calculate the probability of finding a particle in a given part of the box and when simpler “symmetry arguments” can be used. We’ll consider a number of cases where symmetry arguments can be used to specify exactly the probability of ...
... • We will use Slide 12 to determine when calculus must be used to calculate the probability of finding a particle in a given part of the box and when simpler “symmetry arguments” can be used. We’ll consider a number of cases where symmetry arguments can be used to specify exactly the probability of ...
E k
... Si Conduction-Band Structure in wave vector k-space (Constant-Energy Surfaces in k-space)Effective mass approximation: Kinetic energy ...
... Si Conduction-Band Structure in wave vector k-space (Constant-Energy Surfaces in k-space)Effective mass approximation: Kinetic energy ...
The Trouble with Gravity Summary/Review
... – We don’t have a complete understanding of all the sources of the vacuum energy density, but if we look at the quantum fluctuations from any one field, it generates a energy density 120 orders of magnitude larger than the observed value. This implies that the various sources of Λ conspire to cancel ...
... – We don’t have a complete understanding of all the sources of the vacuum energy density, but if we look at the quantum fluctuations from any one field, it generates a energy density 120 orders of magnitude larger than the observed value. This implies that the various sources of Λ conspire to cancel ...
Bloch Oscillations in cold atoms
... • A wave packet with a well defined q in the nth band n /2 F where n is the energy ...
... • A wave packet with a well defined q in the nth band n /2 F where n is the energy ...
Posttest for Uncertainty Principle Part 1
... 1. Ignore normalization issues pertaining to the wave function. At time t=0, the wave packet of a quantum mechanical particle is highly peaked and can be effectively described by a delta function (x) . Is the momentum of this particle well-defined at t=0? Is the position of the particle well-defin ...
... 1. Ignore normalization issues pertaining to the wave function. At time t=0, the wave packet of a quantum mechanical particle is highly peaked and can be effectively described by a delta function (x) . Is the momentum of this particle well-defined at t=0? Is the position of the particle well-defin ...
bukalov02_en
... density is ρv ≈ 2.2∙1071GeV4. This value is in 10120 times more than the observed one ρv(obs) ≈ (2∙10-3eV)4. It is possible to refer to such directions the attempts to solve the cosmological constant problem by analogy to the superconductivity theory. Let's consider the condensation of fermion gas w ...
... density is ρv ≈ 2.2∙1071GeV4. This value is in 10120 times more than the observed one ρv(obs) ≈ (2∙10-3eV)4. It is possible to refer to such directions the attempts to solve the cosmological constant problem by analogy to the superconductivity theory. Let's consider the condensation of fermion gas w ...
Document
... • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can have both wave and particle properties. ...
... • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can have both wave and particle properties. ...