Unit 7 Chap. 7 Chemical Formulas and Compounds
... THE “OLD SYSTEM” OF NAMING BINARY MOLECULAR COMPOUNDS THE GENERAL RULE IS TO NAME THE LESS ELECTRONEGATIVE ELEMENT FIRST AND THE MORE ELECTRONEGATIVE ELEMENT SECOND. THE FIRST WORD OF A BINARY MOLECULAR COMPOUND IS MADE UP OF (A) THE PREFIX OF THE NUMBER OF ATOMS OF THE FIRST ELEMENT AND (B) THE NAM ...
... THE “OLD SYSTEM” OF NAMING BINARY MOLECULAR COMPOUNDS THE GENERAL RULE IS TO NAME THE LESS ELECTRONEGATIVE ELEMENT FIRST AND THE MORE ELECTRONEGATIVE ELEMENT SECOND. THE FIRST WORD OF A BINARY MOLECULAR COMPOUND IS MADE UP OF (A) THE PREFIX OF THE NUMBER OF ATOMS OF THE FIRST ELEMENT AND (B) THE NAM ...
unit 8 – compound stoichiometry
... $7.81/Troy ounce. How much of a profit will they make on this fund-raiser? ...
... $7.81/Troy ounce. How much of a profit will they make on this fund-raiser? ...
Ch. 3 9-Station Review
... Station 9 – CHEMICAL ANALYSIS Solve the following problem: A compound composed of carbon and hydrogen is analyzed by combustion. When a 4.297 g sample of the compound is burned, 12.57 g CO2 and 7.72 g H2O are formed. What is the empirical formula of the compound? ________________ ...
... Station 9 – CHEMICAL ANALYSIS Solve the following problem: A compound composed of carbon and hydrogen is analyzed by combustion. When a 4.297 g sample of the compound is burned, 12.57 g CO2 and 7.72 g H2O are formed. What is the empirical formula of the compound? ________________ ...
Chemical Equilibrium
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
POWERPOINT - Chapter 8
... Divide each molar quantity by the smaller number of moles to get 1 mol for the element with the smaller number of moles. ...
... Divide each molar quantity by the smaller number of moles to get 1 mol for the element with the smaller number of moles. ...
Introduction - CNC Science
... The empirical formula of a compound indicates what elements are present in the compound and the simplest whole number ratio in which the atoms of these elements are present ...
... The empirical formula of a compound indicates what elements are present in the compound and the simplest whole number ratio in which the atoms of these elements are present ...
Chemical Formulas and Composition Stoichiometry
... Writing Formulas and Names for Ionic Compounds (Table 2-3) • Positive and negative ions will combine in such a way to make the ionic compound _____. • Binary Ionic Compounds – You already know how to write the formulas. • Write the formula for calcium bromide and lithium sulfide ...
... Writing Formulas and Names for Ionic Compounds (Table 2-3) • Positive and negative ions will combine in such a way to make the ionic compound _____. • Binary Ionic Compounds – You already know how to write the formulas. • Write the formula for calcium bromide and lithium sulfide ...
AP Chemistry Summer Assignment
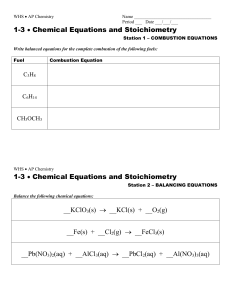
... d. __ Fe2(SO4)3 + __ HCl __ FeCl3+ __ H2O + __ SO3 e. __ C12H24 + __ O2 __ CO2 + __ H2O f. __ Al + __O2 __ Al2O3 g. __ C9H16 + __ O2 __ CO2 + __ H2O h. __ Cr(SO3)2 + __ H2 __ SO2 + __ Cr + __ H2O i. __ C4H8O4 + __ O2 __ CO2 + __ H2O. 41. Define limiting reagent, theoretical yield, and ac ...
... d. __ Fe2(SO4)3 + __ HCl __ FeCl3+ __ H2O + __ SO3 e. __ C12H24 + __ O2 __ CO2 + __ H2O f. __ Al + __O2 __ Al2O3 g. __ C9H16 + __ O2 __ CO2 + __ H2O h. __ Cr(SO3)2 + __ H2 __ SO2 + __ Cr + __ H2O i. __ C4H8O4 + __ O2 __ CO2 + __ H2O. 41. Define limiting reagent, theoretical yield, and ac ...
Chem 115 POGIL Worksheet
... Reactants are written on the left, and products are written on the right. In a balanced equation the total numbers of atoms of each kind on both sides are the same. To achieve a balance, we write coefficients in front of each chemical species, although the number 1 is never written as a coefficient. ...
... Reactants are written on the left, and products are written on the right. In a balanced equation the total numbers of atoms of each kind on both sides are the same. To achieve a balance, we write coefficients in front of each chemical species, although the number 1 is never written as a coefficient. ...
Level 3 Distance Learning
... consequent on the differing phenomena leading to definition of a “frequency” (cm-1) axis in each case. You should consider which bond(s) (metal to carbon, carbon to oxygen) in a carbonyl compound each spectroscopy is powerful in detecting.] (c) Compare and contrast the phenomena that lead to the spe ...
... consequent on the differing phenomena leading to definition of a “frequency” (cm-1) axis in each case. You should consider which bond(s) (metal to carbon, carbon to oxygen) in a carbonyl compound each spectroscopy is powerful in detecting.] (c) Compare and contrast the phenomena that lead to the spe ...
Chapter 06 Notes (PowerPoint) File
... • the relative weights of molecules can be calculated from atomic weights Formula Mass = 1 molecule of H2O = 2(1.01 amu H) + 16.00 amu O = 18.02 amu • since 1 mole of H2O contains 2 moles of H and 1 mole of O Molar Mass = 1 mole H2O = 2(1.01 g H) + 16.00 g O = 18.02 g ...
... • the relative weights of molecules can be calculated from atomic weights Formula Mass = 1 molecule of H2O = 2(1.01 amu H) + 16.00 amu O = 18.02 amu • since 1 mole of H2O contains 2 moles of H and 1 mole of O Molar Mass = 1 mole H2O = 2(1.01 g H) + 16.00 g O = 18.02 g ...
Moles and Stoichiometry
... the empirical mass. This will give you a whole-number multiple that tells you how many times larger the molecular formula is than the empirical formula (54.0 g/mol) / (27.0 g/mol) = 2 ...
... the empirical mass. This will give you a whole-number multiple that tells you how many times larger the molecular formula is than the empirical formula (54.0 g/mol) / (27.0 g/mol) = 2 ...
Midterm exams I
... 4. Where would be Raman lines (in cm-1 ) for (1) diamond and (2) for helium? 5. Which molecule would have higher vibrational frequency: Fluorine (F2) or iodine (I2)? 6. Why Raman spectra should be measured at very low laser power? 7. Why Raman lines of amorphous phase looks broad in comparison to li ...
... 4. Where would be Raman lines (in cm-1 ) for (1) diamond and (2) for helium? 5. Which molecule would have higher vibrational frequency: Fluorine (F2) or iodine (I2)? 6. Why Raman spectra should be measured at very low laser power? 7. Why Raman lines of amorphous phase looks broad in comparison to li ...
Physical concept of the surface tension of the liquid until some time
... A conclusion of this «unpacking» model is that of a reliable agreement with the molecular - kinetic theory of ideal gases. Namely, the theoretical development of the relationship of heat capacity values for one-, two - and triatomic gases. An impact of the spatial arrangement of atoms on the heat ca ...
... A conclusion of this «unpacking» model is that of a reliable agreement with the molecular - kinetic theory of ideal gases. Namely, the theoretical development of the relationship of heat capacity values for one-, two - and triatomic gases. An impact of the spatial arrangement of atoms on the heat ca ...
Chapter 6 Chemical Composition
... The amount of sodium in sodium chloride for diet. The amount of iron in iron ore for steel production. The amount of hydrogen in water for hydrogen fuel. The amount of chlorine in freon to estimate ozone ...
... The amount of sodium in sodium chloride for diet. The amount of iron in iron ore for steel production. The amount of hydrogen in water for hydrogen fuel. The amount of chlorine in freon to estimate ozone ...
AP Chemistry Syllabus 2013 Mawhiney
... 2. Use experimental data to determine the rate law, determine the order of the reaction, and to define proper units for the constant. 3. Compare and contrast zero, first, and second order reactions in terms of the plot needed to give a straight line, the relationship of the rate constant to the slop ...
... 2. Use experimental data to determine the rate law, determine the order of the reaction, and to define proper units for the constant. 3. Compare and contrast zero, first, and second order reactions in terms of the plot needed to give a straight line, the relationship of the rate constant to the slop ...
Spring 2009 Final Exam Review – Part 2
... Each orbital is associated with a specific amount of energy. When an electron absorbs energy from an outside source, it jumps up energy levels to an excited state. The electron then releases that energy in order to move back down into ins ground state. Sometimes, that energy is released as visible l ...
... Each orbital is associated with a specific amount of energy. When an electron absorbs energy from an outside source, it jumps up energy levels to an excited state. The electron then releases that energy in order to move back down into ins ground state. Sometimes, that energy is released as visible l ...
Topic 4 Formulae, Equations and Mole
... Step 1: Start by finding out how many atoms of each type are on each side of the equation. (Some teachers recommend making a little table listing the numbers of each atom for the left hand side and for the right hand side.) Step 2: Next, look for an element which is in only one chemical on the left ...
... Step 1: Start by finding out how many atoms of each type are on each side of the equation. (Some teachers recommend making a little table listing the numbers of each atom for the left hand side and for the right hand side.) Step 2: Next, look for an element which is in only one chemical on the left ...
Setting the stage
... Infrared spectroscopy utilized in chemical simulations of reactions on interstellar ices. Various studies have been performed on amphiphilic vesicle forming molecules. ...
... Infrared spectroscopy utilized in chemical simulations of reactions on interstellar ices. Various studies have been performed on amphiphilic vesicle forming molecules. ...
1) abcde 2) abcde 3) abcde 4) abcde 5) abcde 6) abcde 7) abcde 8
... 9) When a chemical reaction takes place, which of the following are conserved? 1 - number of atoms 2 - number of molecules 3 - number of moles 4 - mass 5 - volume (a) 1, 3 (b) 2, 4 (c) 3, 5 (d) 1, 4 (e) 2, 5 10) The number of moles of CO2 in 0.66 g of the gas is (a) 0.015 (b) 0. (c) 0.44 (d) 2.2 11) ...
... 9) When a chemical reaction takes place, which of the following are conserved? 1 - number of atoms 2 - number of molecules 3 - number of moles 4 - mass 5 - volume (a) 1, 3 (b) 2, 4 (c) 3, 5 (d) 1, 4 (e) 2, 5 10) The number of moles of CO2 in 0.66 g of the gas is (a) 0.015 (b) 0. (c) 0.44 (d) 2.2 11) ...
The Future is Noisy: The Role of Spatial Fluctuations
... However, we will show that the involved system parameters are not consistent with the above reasoning, and that well stirredness alone is not sufficient to obtain a complete picture of the process. In contrast, we give evidence that the overall process consists of a large number of subprocesses duri ...
... However, we will show that the involved system parameters are not consistent with the above reasoning, and that well stirredness alone is not sufficient to obtain a complete picture of the process. In contrast, we give evidence that the overall process consists of a large number of subprocesses duri ...
Host–guest chemistry

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through noncovalent bonding. Noncovalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. There are four commonly mentioned types of non-covalent interactions: hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions.