guess paper class xii
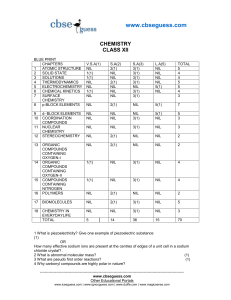
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
phenols - Gneet`s
... The greater acidity of phenol is due to stability of phenoxide ion is resonance stabilised . ...
... The greater acidity of phenol is due to stability of phenoxide ion is resonance stabilised . ...
Chemistry IGCSE
... so quickly that they form bubbles of gas in the liquid. This is the boiling point at which the pressure of the gas forming above the liquid is the same as atmospheric pressure. On the other hand, cooling a gas will make its particles lose their kinetic energy and move closer and slower. Eventually t ...
... so quickly that they form bubbles of gas in the liquid. This is the boiling point at which the pressure of the gas forming above the liquid is the same as atmospheric pressure. On the other hand, cooling a gas will make its particles lose their kinetic energy and move closer and slower. Eventually t ...
Document
... • The natural state of an element is the most stable state (gas, liquid or solid) of that element, i.e., the state at which that element is found in nature. • Standard reference state conditions: Tref = 25 ºc, pref=po = 1 atm (superscript denotes atmospheric pressure) ...
... • The natural state of an element is the most stable state (gas, liquid or solid) of that element, i.e., the state at which that element is found in nature. • Standard reference state conditions: Tref = 25 ºc, pref=po = 1 atm (superscript denotes atmospheric pressure) ...
N Goalby chemrevise.org 1 2.5 Transition Metals Substitution
... The copper complex ion has changed from having unidentate ligands to a multidentate ligand. In this reaction there is an increase in the entropy because there are more moles of products than reactants (from 2 to 7), creating more disorder. The enthalpy change is small as there are similar numbers of ...
... The copper complex ion has changed from having unidentate ligands to a multidentate ligand. In this reaction there is an increase in the entropy because there are more moles of products than reactants (from 2 to 7), creating more disorder. The enthalpy change is small as there are similar numbers of ...
Chemical Quantities: Stoichiometry and the Mole
... you follow the steps you can get to the answer. Think about the “steps” I mentioned when we talked about problem solving. When we learned how to write the names of compounds for their formulas, and the formulas for the names. When we do conversions and stoichiometry. I am half cliche when I describe ...
... you follow the steps you can get to the answer. Think about the “steps” I mentioned when we talked about problem solving. When we learned how to write the names of compounds for their formulas, and the formulas for the names. When we do conversions and stoichiometry. I am half cliche when I describe ...
GCE Getting Started - Edexcel
... After KS4 exams – if your school brings back Yr11 learners after their exams. ...
... After KS4 exams – if your school brings back Yr11 learners after their exams. ...
Ignition Processes in Hydrogen
... detailed reaction mechanism and a mUltispecies transport model. An additional source term in the energy conservation allowed the treatment of induced ignition, and a realistic model for the destruction of reactive species at the vessel surface was used to treat auto-ignitions in static reactors. Spa ...
... detailed reaction mechanism and a mUltispecies transport model. An additional source term in the energy conservation allowed the treatment of induced ignition, and a realistic model for the destruction of reactive species at the vessel surface was used to treat auto-ignitions in static reactors. Spa ...
Lab # 18
... 8. What is a diatomic molecule? 9. What phase are most diatomic molecules found in? 10. What is a chemical equation? 11. How can you tell whether an equation is balanced? 12. What can an equation tell us about bonding? Problem: How can we use molecular models to help us balance equations? Introducti ...
... 8. What is a diatomic molecule? 9. What phase are most diatomic molecules found in? 10. What is a chemical equation? 11. How can you tell whether an equation is balanced? 12. What can an equation tell us about bonding? Problem: How can we use molecular models to help us balance equations? Introducti ...
Kinetic Control of Aqueous Polymerization Using Radicals
... by free radical encounters. These spin states describe different electron configurations. Depending on these configurations the caged radical pair may recombine regenerating the initial molecule, undergoing the formation of cage products which generates a new molecule, or the radicals can escape fro ...
... by free radical encounters. These spin states describe different electron configurations. Depending on these configurations the caged radical pair may recombine regenerating the initial molecule, undergoing the formation of cage products which generates a new molecule, or the radicals can escape fro ...
Potassium trans
... from biological material [28]. Here, similar small gas molecule uptake reactions have been investigated for the title complex to and their reaction mechanism has been determined. Investigations involving cobalt(II) complexes with oxalates can provide information about their interesting kinetic and a ...
... from biological material [28]. Here, similar small gas molecule uptake reactions have been investigated for the title complex to and their reaction mechanism has been determined. Investigations involving cobalt(II) complexes with oxalates can provide information about their interesting kinetic and a ...
CHEM 210 IR Spectroscopy
... 1. Each stretching and bending vibration occurs with a characteristic frequency as the atoms and charges involved are different for different bonds The y-axis on an IR spectrum is in units of % transmittance In regions where the EM field of an osc. bond interacts with IR light of the same n – transm ...
... 1. Each stretching and bending vibration occurs with a characteristic frequency as the atoms and charges involved are different for different bonds The y-axis on an IR spectrum is in units of % transmittance In regions where the EM field of an osc. bond interacts with IR light of the same n – transm ...
Chapter 5: Thermochemistry
... 5.7 Heat Transfer and Thermal Equilibrium: Simulation When an object is heated, three things can happen: it can get warmer, it can undergo a phase change, and it can undergo a chemical change. In this section, we explore the first two possibilities while the remainder of the chapter explores the thi ...
... 5.7 Heat Transfer and Thermal Equilibrium: Simulation When an object is heated, three things can happen: it can get warmer, it can undergo a phase change, and it can undergo a chemical change. In this section, we explore the first two possibilities while the remainder of the chapter explores the thi ...
18.3 Standard Entropies and the Third Law of
... nonspontaneous* describes a physical or chemical change that occurs only with the aid of an outside force or agent (18.2, introductory section) entropy (S) thermodynamic quantity that is a measure of how dispersed the energy of a system is among the different possible ways that system can contain en ...
... nonspontaneous* describes a physical or chemical change that occurs only with the aid of an outside force or agent (18.2, introductory section) entropy (S) thermodynamic quantity that is a measure of how dispersed the energy of a system is among the different possible ways that system can contain en ...