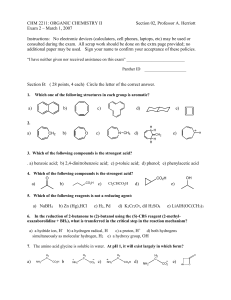
(1) Dissolves, accompanied by evolution of flammable gas (2
... 5. Consider the molecules PF3 and PF5. (a) Draw the Lewis electron-dot structures for PF3 and PF5 and predict the molecular geometry of each. (b) Is the PF3 molecule polar, or is it nonpolar? Explain. (c) On the basis of bonding principles, predict whether each of the following compounds exists. In ...
... 5. Consider the molecules PF3 and PF5. (a) Draw the Lewis electron-dot structures for PF3 and PF5 and predict the molecular geometry of each. (b) Is the PF3 molecule polar, or is it nonpolar? Explain. (c) On the basis of bonding principles, predict whether each of the following compounds exists. In ...
Unit 2 Study Guide - Alexander`s 8th Grade Physical Science
... 26. What is the definition of a physical change? List some examples. • A physical change is when a substance undergoes a change in some properties but keeps MOST of its properties, and does NOT create a new substance. Examples: cutting paper, breaking a mirror. ...
... 26. What is the definition of a physical change? List some examples. • A physical change is when a substance undergoes a change in some properties but keeps MOST of its properties, and does NOT create a new substance. Examples: cutting paper, breaking a mirror. ...
Review 3rd Qtr KEY
... 13. Explain why chromium’s electron configuration is [Ar] 4s13d5 instead of the expected configuration of [Ar] 4s23d4 Filled & ½ filled orbital’s are more stable. 14. Complete the following question based upon Cobalt (#27) a) Give the noble gas electron configuration for this element: ______________ ...
... 13. Explain why chromium’s electron configuration is [Ar] 4s13d5 instead of the expected configuration of [Ar] 4s23d4 Filled & ½ filled orbital’s are more stable. 14. Complete the following question based upon Cobalt (#27) a) Give the noble gas electron configuration for this element: ______________ ...
Review of Definitions
... another atom in a chemical bond. The electronegativity of an atom depends on two factors : (1) the number of positive charges in the nucleus (the more protons, the more electronegative) and (2) the distance of the outer electrons from the nucleus (the greater the distance, the less electronegative) ...
... another atom in a chemical bond. The electronegativity of an atom depends on two factors : (1) the number of positive charges in the nucleus (the more protons, the more electronegative) and (2) the distance of the outer electrons from the nucleus (the greater the distance, the less electronegative) ...
Word - chemmybear.com
... Given a formula, name the molecule. Given a name, write the correct formula. ...
... Given a formula, name the molecule. Given a name, write the correct formula. ...
urbano, mariajose
... Holism: The principle that a higher level of order cannot be meaningfully explained by examining component parts in isolation. • An organism is a living whole greater than the sum of its parts. • For example, a cell dismantled to its chemical ingredients is no longer a cell. 4. Describe seven emerge ...
... Holism: The principle that a higher level of order cannot be meaningfully explained by examining component parts in isolation. • An organism is a living whole greater than the sum of its parts. • For example, a cell dismantled to its chemical ingredients is no longer a cell. 4. Describe seven emerge ...
Exam 2
... 6. In the reduction of 2-butanone to (2)-butanol using the (S)-CBS reagent (2-methyloxazaborolidine + BH3), what is transferred in the critical step in the reaction mechanism? a) a hydride ion, H- b) a hydrogen radical, H c) a proton, H+ d) both hydrogens simultaneously as molecular hydrogen, H2 e) ...
... 6. In the reduction of 2-butanone to (2)-butanol using the (S)-CBS reagent (2-methyloxazaborolidine + BH3), what is transferred in the critical step in the reaction mechanism? a) a hydride ion, H- b) a hydrogen radical, H c) a proton, H+ d) both hydrogens simultaneously as molecular hydrogen, H2 e) ...
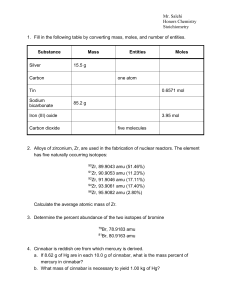
Stoichiometry - Cloudfront.net
... 5. Acetaminophen has the molecular formula C8H9NO2. a. What is the mass % of each element in acetaminophen? b. How many grams of carbon are in a 1.41 g sample of acetaminophen? 6. A 2.074 g sample of an unknown compound was subjected to combustion analysis, and produced 3.800 g of CO2 and 1.040 g of ...
... 5. Acetaminophen has the molecular formula C8H9NO2. a. What is the mass % of each element in acetaminophen? b. How many grams of carbon are in a 1.41 g sample of acetaminophen? 6. A 2.074 g sample of an unknown compound was subjected to combustion analysis, and produced 3.800 g of CO2 and 1.040 g of ...
SNC2D – Science 10 Tuesday April 26th, 2010 Mr. Sourlis and Mr
... e. gold 2. Which one of the following elements has 5 valence electrons? a. boron b. lithium c. hydrogen d. iodine e. phosphorus 3. When a chemical reaction takes place, the total mass of the products is always a. Greater than the total mass of the reactants b. Less than the total mass of the reactan ...
... e. gold 2. Which one of the following elements has 5 valence electrons? a. boron b. lithium c. hydrogen d. iodine e. phosphorus 3. When a chemical reaction takes place, the total mass of the products is always a. Greater than the total mass of the reactants b. Less than the total mass of the reactan ...
ORGANIC CHEMISTRY BASICS
... Date_________ Period_______ OBJECTIVE: To be able to assemble models of several simple hydrocarbons and relate the 3-D shapes of molecules to their names and structural formulas used to represent them on paper. PART 1 – STRAIGHT CHAIN ALKANES PROCEDURE: 1. Assemble a 3-D molecular model of methane ( ...
... Date_________ Period_______ OBJECTIVE: To be able to assemble models of several simple hydrocarbons and relate the 3-D shapes of molecules to their names and structural formulas used to represent them on paper. PART 1 – STRAIGHT CHAIN ALKANES PROCEDURE: 1. Assemble a 3-D molecular model of methane ( ...
The student will
... require students to work with equipment they would not normally see in a college-preparatory class. They perform extended labs running multiple trials and analyzing results in the same way one would be expected to in college. Students work in pairs and frequently discuss results, validity, and possi ...
... require students to work with equipment they would not normally see in a college-preparatory class. They perform extended labs running multiple trials and analyzing results in the same way one would be expected to in college. Students work in pairs and frequently discuss results, validity, and possi ...
CHE 106, F`95 E1(Word)
... The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? (a) (b) (c) (d) (e) ...
... The sum of the masses of two hydrogen atoms (mass number 1) and two neutrons is 4.0330. Why does this differ from the mass of a helium atom (4.0026)? (a) (b) (c) (d) (e) ...
Reactions Homework Packet
... 1. Calculate the oxidation state of each element in each of the following: ...
... 1. Calculate the oxidation state of each element in each of the following: ...