Balancing chemical equations notes
... Reactants are the chemicals that are initially combined together. They are written on the left side of the reaction arrow. In the case above, Cu and HNO3 are reactants. Products are the chemicals produced by a reaction. They are written on the right side of the reaction arrow. In the case above, Cu( ...
... Reactants are the chemicals that are initially combined together. They are written on the left side of the reaction arrow. In the case above, Cu and HNO3 are reactants. Products are the chemicals produced by a reaction. They are written on the right side of the reaction arrow. In the case above, Cu( ...
Eötvös Loránd Science University Faculty of Sciences Department of
... Historical review of chemical kinetics. The scope of modern kinetics. Definition of the reaction rate and its formulation using different time derivatives. Collision theory in kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Al ...
... Historical review of chemical kinetics. The scope of modern kinetics. Definition of the reaction rate and its formulation using different time derivatives. Collision theory in kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Al ...
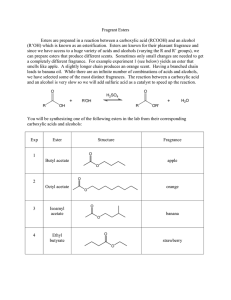
Fragrant Esters Esters are prepared in a reaction between a
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
Chapter 25 Organic and Biological Chemistry
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
... Reactions of Aromatic Compounds • Unlike in alkenes and alkynes, electrons do not sit between two atoms. • Electrons are delocalized; this stabilizes aromatic compounds. Organic and Biological Chemistry ...
1st Semester Exam in High School Chemistry
... 7. Which of the following BEST describes what happens when most substances change from a solid state to a liquid state? A. The molecules slow down. B. The molecules move farther apart. C. The molecules get smaller. D. The molecules lose energy. ...
... 7. Which of the following BEST describes what happens when most substances change from a solid state to a liquid state? A. The molecules slow down. B. The molecules move farther apart. C. The molecules get smaller. D. The molecules lose energy. ...
Chemical Reactions
... Ionic Equations • A _______ ionic equation shows only those particles involved in the reaction and is balanced with respect to both __________ and ____________. • You can predict the formation of a ______________ by using the general rules for solubility of ionic compounds. ...
... Ionic Equations • A _______ ionic equation shows only those particles involved in the reaction and is balanced with respect to both __________ and ____________. • You can predict the formation of a ______________ by using the general rules for solubility of ionic compounds. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 4. Write the Woodward Hoffmann rules for electrocyclization reaction. 5. Explain the change in the geometry of excited state molecule in a photochemical process and the variation in its physical property. 6. What is Norrish type I and II cleavage reactions? Give suitable examples. 7. What are the im ...
... 4. Write the Woodward Hoffmann rules for electrocyclization reaction. 5. Explain the change in the geometry of excited state molecule in a photochemical process and the variation in its physical property. 6. What is Norrish type I and II cleavage reactions? Give suitable examples. 7. What are the im ...
Measuring and Calculating
... the process of reacting a stoichiometric amount of base with an acid; when done with a strong acid and base this will produce a salt and water ...
... the process of reacting a stoichiometric amount of base with an acid; when done with a strong acid and base this will produce a salt and water ...
Scientific Notation - Warren County Public Schools
... • Nitrogen is a neutral atom. It has 7neutrons and ...
... • Nitrogen is a neutral atom. It has 7neutrons and ...
Lecture 6
... 4 atoms of iron react with 3 molecules of oxygen to produce 2 molecules of iron(III) oxide This equation can be read in “moles” by placing the words “moles of” between each coefficient and formula. 4 moles of Fe + 3 moles of O2 ...
... 4 atoms of iron react with 3 molecules of oxygen to produce 2 molecules of iron(III) oxide This equation can be read in “moles” by placing the words “moles of” between each coefficient and formula. 4 moles of Fe + 3 moles of O2 ...
Chemistry EOC Review
... 104. How are the pressure and volume of a gas related? 105. A gas is originally at a volume of 6 mL and a pressure of 1 atm. If the pressure is increased to 2 atm, what is the new volume of the gas? 106. State Charles’s Law (*Remember that temperature in Charles’s Law must be in Kelvin) 107. Oxygen ...
... 104. How are the pressure and volume of a gas related? 105. A gas is originally at a volume of 6 mL and a pressure of 1 atm. If the pressure is increased to 2 atm, what is the new volume of the gas? 106. State Charles’s Law (*Remember that temperature in Charles’s Law must be in Kelvin) 107. Oxygen ...
Bonding
... ✦ Electrons are transferred ✦ Metals react with nonmetals ✦ Ions paired have lower energy (greater stability) than separated ions Covalent ✦ Electrons are shared by nuclei ✦ Pure covalent (nonpolar covalent) - electrons are shared evenly ✦ Polar covalent - electrons shared unequally ...
... ✦ Electrons are transferred ✦ Metals react with nonmetals ✦ Ions paired have lower energy (greater stability) than separated ions Covalent ✦ Electrons are shared by nuclei ✦ Pure covalent (nonpolar covalent) - electrons are shared evenly ✦ Polar covalent - electrons shared unequally ...
Chem12 SM Unit 1 Review final new ok revised
... molecule that affect the properties of the compound, such as solubility, melting point, boiling point, and chemical reactivity. Organic molecules are classified according to their functional groups. 99. Methene is not an appropriate name for a compound because the prefix methindicates a single carbo ...
... molecule that affect the properties of the compound, such as solubility, melting point, boiling point, and chemical reactivity. Organic molecules are classified according to their functional groups. 99. Methene is not an appropriate name for a compound because the prefix methindicates a single carbo ...
Chapter 1
... The quantitative nature of chemical formulas and reactions is called stoichiometry. Chemical equations give a description of a chemical reaction. There are two parts to any equation: • reactants (written to the left of the arrow) and • products (written to the right of the arrow): 2H2 + O2 2H2O T ...
... The quantitative nature of chemical formulas and reactions is called stoichiometry. Chemical equations give a description of a chemical reaction. There are two parts to any equation: • reactants (written to the left of the arrow) and • products (written to the right of the arrow): 2H2 + O2 2H2O T ...
Exam 2-Answer Key
... two pi bonds and a sigma bond, each formed by a lateral overlap of two p orbitals. a sigma bond formed by overlap of two s orbitals and two pi bonds, each formed by lateral overlap of two p orbitals. a sigma bond formed by end-on overlap of two sp" orbitals and a pi bond formed by lateral overlap of ...
... two pi bonds and a sigma bond, each formed by a lateral overlap of two p orbitals. a sigma bond formed by overlap of two s orbitals and two pi bonds, each formed by lateral overlap of two p orbitals. a sigma bond formed by end-on overlap of two sp" orbitals and a pi bond formed by lateral overlap of ...
Chemistry Final - Practice Test I
... What was the contribution to chemistry by each of these individuals? Neils Bohr Developed the Planetary Model of the atom based on Quantum energy levels Henry Moseley Arranged the Periodic Table – Increasing atomic number using x-rays and wavelengths Rutherford Discovered that most of the atoms mass ...
... What was the contribution to chemistry by each of these individuals? Neils Bohr Developed the Planetary Model of the atom based on Quantum energy levels Henry Moseley Arranged the Periodic Table – Increasing atomic number using x-rays and wavelengths Rutherford Discovered that most of the atoms mass ...
Chapters 9 and 10
... Indicate the total number of sigma (σ) bonds and the total number of pi (π) bonds in the molecule ...
... Indicate the total number of sigma (σ) bonds and the total number of pi (π) bonds in the molecule ...