Chem 240 - Napa Valley College
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
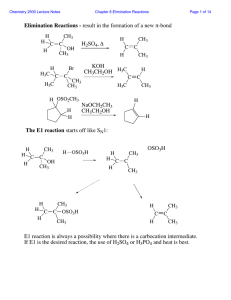
Elimination Reactions - result in the formation of a new π
... In an E2 reaction, a strong base removes a proton beta to the leaving group so long as there exists a conformation in which the proton is anti-periplanar to the leaving group. Where there are several such protons, several products are possible: ...
... In an E2 reaction, a strong base removes a proton beta to the leaving group so long as there exists a conformation in which the proton is anti-periplanar to the leaving group. Where there are several such protons, several products are possible: ...
Amino Acids and Proteins
... The 20 amino acids that occur naturally in proteins differ in the identity of the R group bonded to the α carbon. The R group is called the side chain of the amino acid. The simplest amino acid, called glycine, has R = H. All other amino acids (R ñ H) have a stereogenic center on the ` carbon. As is ...
... The 20 amino acids that occur naturally in proteins differ in the identity of the R group bonded to the α carbon. The R group is called the side chain of the amino acid. The simplest amino acid, called glycine, has R = H. All other amino acids (R ñ H) have a stereogenic center on the ` carbon. As is ...
PHYSICOCHEMICAL PROPERTIES OF ORGANIC MEDICINAL
... Earlier in this tutorial it was noted that alcohols are considered to be relatively non-acidic and nonbasic compounds, while most phenols are classified as very weak acids and non-bases. Thus neither alcohols nor phenols are significantly ionized at physiological pHs (5-8). Also neither alcohols nor ...
... Earlier in this tutorial it was noted that alcohols are considered to be relatively non-acidic and nonbasic compounds, while most phenols are classified as very weak acids and non-bases. Thus neither alcohols nor phenols are significantly ionized at physiological pHs (5-8). Also neither alcohols nor ...
Chemistry - College of LAS
... CHEM 102 General Chemistry I credit: 3 hours. For students who have some prior knowledge of chemistry. Principles governing atomic structure, bonding, states of matter, stoichiometry, and chemical equilibrium. Credit is not given for both CHEM 102 and CHEM 202. CHEM 102 and CHEM 103 are approved for ...
... CHEM 102 General Chemistry I credit: 3 hours. For students who have some prior knowledge of chemistry. Principles governing atomic structure, bonding, states of matter, stoichiometry, and chemical equilibrium. Credit is not given for both CHEM 102 and CHEM 202. CHEM 102 and CHEM 103 are approved for ...
Chemistry of alcohols (powerpoint)
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
... THE CHEMISTRY OF ALCOHOLS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature ru ...
Heterogeneous Catalysts for Biodiesel Production
... (c) pre-esterification method. FFAs are first esterified to FAMEs using an acid catalyst, and then, transesterification is performed, as usual, by using an alkaline catalyst.5,10 Enzyme-based transesterification is carried out at moderate temperatures with high yields, but this method cannot be used ...
... (c) pre-esterification method. FFAs are first esterified to FAMEs using an acid catalyst, and then, transesterification is performed, as usual, by using an alkaline catalyst.5,10 Enzyme-based transesterification is carried out at moderate temperatures with high yields, but this method cannot be used ...
Ch. 6 - Department of Chemistry and Biochemistry
... NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in re ...
... NSERC postdoctoral fellow at the Imperial College (UK) and at Harvard University (USA). He joined the Department of Chemistry at the University of Guelph (Ontario, Canada) in 1998 and is currently a Full Professor and Associate Chair in the department. Professor Tam has received several awards in re ...
PDF of this page - Miami bulletin
... methodology, and skills used in the application of scientific methodology. Credit not given for both CHM 141R and 141. IVB, LAB. CAS-D. Prerequisite: one year of high school chemistry and a math placement score of 12 or higher or permission of instructor. Co-requisite: CHM 144. CHM 141H. College Che ...
... methodology, and skills used in the application of scientific methodology. Credit not given for both CHM 141R and 141. IVB, LAB. CAS-D. Prerequisite: one year of high school chemistry and a math placement score of 12 or higher or permission of instructor. Co-requisite: CHM 144. CHM 141H. College Che ...
Synthetic applications of ortho esters
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
Topic 9 Reduction and Oxidation File
... Recognizing Oxidation-Reduction Reactions Oxidation-reduction reactions are reactions in which one type of atom increases in oxidation number (is oxidized) and another type of atom decreases in oxidation number (is reduced). Thus to show that a reaction is a redox reaction, you need to calculate ox ...
... Recognizing Oxidation-Reduction Reactions Oxidation-reduction reactions are reactions in which one type of atom increases in oxidation number (is oxidized) and another type of atom decreases in oxidation number (is reduced). Thus to show that a reaction is a redox reaction, you need to calculate ox ...
Document
... • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one stereoisomer is formed preferentially. Why? ...
... • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one stereoisomer is formed preferentially. Why? ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... In a covalent bond, electrons are attracted to two nuclei, but sometimes one nucleus attracts the electrons more strongly than the other. When one nucleus attracts the electrons more strongly, the bonding electrons are located closer to one nucleus than the other. This creates an uneven distribution ...
... In a covalent bond, electrons are attracted to two nuclei, but sometimes one nucleus attracts the electrons more strongly than the other. When one nucleus attracts the electrons more strongly, the bonding electrons are located closer to one nucleus than the other. This creates an uneven distribution ...
Procedure - Loudoun County Public Schools
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
JOURNAL OF FLOW CHEMISTRY (ISSN: 2062
... same lipase was immobilised by different methods. Such phenomena were observed when CaLA was adsorbed either on phenyl-grafted silica gel or attached covalently to porous polymethacrylate beads (Entries 6 and 7, respectively) or when CaLB was adsorbed on acrylic resin or on phenyl modified silica ge ...
... same lipase was immobilised by different methods. Such phenomena were observed when CaLA was adsorbed either on phenyl-grafted silica gel or attached covalently to porous polymethacrylate beads (Entries 6 and 7, respectively) or when CaLB was adsorbed on acrylic resin or on phenyl modified silica ge ...