Microwave-Enhanced Sulphated Zirconia and SZ/MCM
... There is a noticeable growing interest in obtaining solid catalysts that should be able to replace those commonly used, such as concentrated H2SO4, HCl and triflates, among others, for chemical transformations because they can be recovered, reused, and are generally innocuous to the environment. Sul ...
... There is a noticeable growing interest in obtaining solid catalysts that should be able to replace those commonly used, such as concentrated H2SO4, HCl and triflates, among others, for chemical transformations because they can be recovered, reused, and are generally innocuous to the environment. Sul ...
Common Chemical Formula List
... with chemical symbols, as then you will be able to see how many atoms of each type are in each chemical. Example 1 Unbalanced Equation:- C3H8 + O2 ---> H2O + CO2 There are three carbons on the left, but only one on the right. There are eight hydrogens on the left but only two on the right. There are ...
... with chemical symbols, as then you will be able to see how many atoms of each type are in each chemical. Example 1 Unbalanced Equation:- C3H8 + O2 ---> H2O + CO2 There are three carbons on the left, but only one on the right. There are eight hydrogens on the left but only two on the right. There are ...
10.3 Ligand Field Theory 10.3 Ligand Field Theory
... - (b): ligands w/ e- in p orbitals (e.g. F-, Cl-) bonding molecular π orbitals: occupied by these e- ...
... - (b): ligands w/ e- in p orbitals (e.g. F-, Cl-) bonding molecular π orbitals: occupied by these e- ...
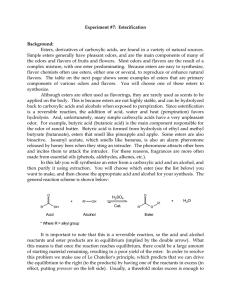
102 Lab 7 Esters Fall05
... the odors and flavors of fruits and flowers. Most odors and flavors are the result of a complex mixture, with one ester predominating. Because esters are easy to synthesize, flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page s ...
... the odors and flavors of fruits and flowers. Most odors and flavors are the result of a complex mixture, with one ester predominating. Because esters are easy to synthesize, flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page s ...
AP Chapter Five Outline
... When ionic compounds dissolve in water, they dissociate into ions surrounded by water molecules. An ionic compound that completely dissolves into ions is a strong electrolyte. A. Exchange Reactions: AB + CD AD + CB 1. If both reactants and products are water-soluble compounds, then no overall re ...
... When ionic compounds dissolve in water, they dissociate into ions surrounded by water molecules. An ionic compound that completely dissolves into ions is a strong electrolyte. A. Exchange Reactions: AB + CD AD + CB 1. If both reactants and products are water-soluble compounds, then no overall re ...
How to Make a Collage
... The use of a calculator will be kept to a minimum. The AP exam only allows you to use the calculator on certain sections. Thus, your expertise in math is a necessity. You can expect to utilize basic math skills that you began to learn in elementary school. Addition, subtraction, multiplication and d ...
... The use of a calculator will be kept to a minimum. The AP exam only allows you to use the calculator on certain sections. Thus, your expertise in math is a necessity. You can expect to utilize basic math skills that you began to learn in elementary school. Addition, subtraction, multiplication and d ...
NZIC 2012 - Rangiora High School
... means there are more HCl molecules per unit volume to react at any one time. This increases the total number of collisions per second (frequency of collisions) to give a faster rate of reaction. ...
... means there are more HCl molecules per unit volume to react at any one time. This increases the total number of collisions per second (frequency of collisions) to give a faster rate of reaction. ...
Packet #7- Chemical Reactions
... Conservation of mass [E] No atoms are lost or made during a chemical reaction. This means that the mass is always conserved. In other words, the total mass of products after the reaction is the same as the total mass of the reactants at the start. This fact allows you to work out the mass of one sub ...
... Conservation of mass [E] No atoms are lost or made during a chemical reaction. This means that the mass is always conserved. In other words, the total mass of products after the reaction is the same as the total mass of the reactants at the start. This fact allows you to work out the mass of one sub ...
AP Chemistry - Forsyth County Schools
... A review book is highly recommended, though not required. Princeton Review, and/or 5 steps to a 5 are all good for different reasons. Barrons is also an option, but can be a bit overwhelming Supplies during the school year Lab notebook WebAssign subscription Scientific or graphing calculator ...
... A review book is highly recommended, though not required. Princeton Review, and/or 5 steps to a 5 are all good for different reasons. Barrons is also an option, but can be a bit overwhelming Supplies during the school year Lab notebook WebAssign subscription Scientific or graphing calculator ...
Tutorial 6 Writing Chemical Formulas for Molecular Compounds and
... • Ionic compounds form when metals transfer valence electrons to nonmetals forming cations and anions, respectively. The ratio of cations to anions is always expressed in the simplest whole number ratio k ...
... • Ionic compounds form when metals transfer valence electrons to nonmetals forming cations and anions, respectively. The ratio of cations to anions is always expressed in the simplest whole number ratio k ...
PRE AP CHEMISTRY REVIEW PROBLEMS NON COLLEGE
... g. How many molecules are in a 1.20 mol sample of oxygen gas? How many oxygen atoms are in this sample? h. How many moles are in a 75.4 g sample of sulfur tetrachloride? i. What is the volume at STP of a 102 g sample of propane gas, C3H8? j. What is the mass of a sample that contains 1.22 × 1022 arg ...
... g. How many molecules are in a 1.20 mol sample of oxygen gas? How many oxygen atoms are in this sample? h. How many moles are in a 75.4 g sample of sulfur tetrachloride? i. What is the volume at STP of a 102 g sample of propane gas, C3H8? j. What is the mass of a sample that contains 1.22 × 1022 arg ...
The Sabatier Principle Illustrated by Catalytic H2
... combining a theoretical model with experiments to gain an understanding of the differences in catalytic activity of relevant materials via the Sabatier principle. Furthermore, this approach gives the students hands-on lab experience in a simple and fun experiment, which can be interpreted qualitative ...
... combining a theoretical model with experiments to gain an understanding of the differences in catalytic activity of relevant materials via the Sabatier principle. Furthermore, this approach gives the students hands-on lab experience in a simple and fun experiment, which can be interpreted qualitative ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numbering 5. if more than 1 subst., place all in alphabetical order in front 6. if counting from either end is tie in yne #, us ...
... 3. if 2 triple bonds, diyne a. if 3 trple bonds, triyne b. if 4, tetrayne, then pentayne, hexayne, heptayne, etc 4. with number (#) as prefix a. funct grp has priority with numbering 5. if more than 1 subst., place all in alphabetical order in front 6. if counting from either end is tie in yne #, us ...
Chemical Equations
... Reaction Types: Synthesis or Composition • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction ...
... Reaction Types: Synthesis or Composition • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction ...
File
... Which Group 15 element exists as diatomic molecules at STP? (1) phosphorus (2) nitrogen (3) bismuth (4) arsenic Which substance can not be broken down by a chemical change? (1) methane (2) propanal (3) tungsten (4) water Which unit can be used to express the concentration of a solution? (1) L/s (2) ...
... Which Group 15 element exists as diatomic molecules at STP? (1) phosphorus (2) nitrogen (3) bismuth (4) arsenic Which substance can not be broken down by a chemical change? (1) methane (2) propanal (3) tungsten (4) water Which unit can be used to express the concentration of a solution? (1) L/s (2) ...
Chapter 1 Review, pages 72–77
... 37. (a) The compound on the right, benzoic acid, has two polar groups—a carbonyl group and a hydroxyl group—located close together, adding polarity to the molecule, which contributes to its solubility in water. However, the non-polar ring makes benzoic acid less soluble. Consequently, benzoic acid ...
... 37. (a) The compound on the right, benzoic acid, has two polar groups—a carbonyl group and a hydroxyl group—located close together, adding polarity to the molecule, which contributes to its solubility in water. However, the non-polar ring makes benzoic acid less soluble. Consequently, benzoic acid ...
AS Chemistry - Crawshaw Academy
... AS Chemistry is an academically demanding course. Prospective students should be comfortable with the basic Chemistry from the GCSE course, most significantly: ‘Bonding and Structure’, ‘Periodicity’, ‘Chemical Formulae’, Chemistry Calculations’ and ‘Balancing Equations’. In order for you to settle i ...
... AS Chemistry is an academically demanding course. Prospective students should be comfortable with the basic Chemistry from the GCSE course, most significantly: ‘Bonding and Structure’, ‘Periodicity’, ‘Chemical Formulae’, Chemistry Calculations’ and ‘Balancing Equations’. In order for you to settle i ...
CHM 212 - The Federal University of Agriculture, Abeokuta
... under standard condition. It is the enthalpy change that accompanied the formation of one mole when one mole of an ionic crystal is formed from its constituent ions in the gaseous state under standard conditions. It is a measure of ionic strength. Lattice energy( ies) cannot be measured directly, bu ...
... under standard condition. It is the enthalpy change that accompanied the formation of one mole when one mole of an ionic crystal is formed from its constituent ions in the gaseous state under standard conditions. It is a measure of ionic strength. Lattice energy( ies) cannot be measured directly, bu ...
Energy of Reactions
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
Chapter 13…States of Matter
... 17. (Ionic/molecular) compounds cause greater change of boiling/freezing points in solutions. 18. Aluminum chloride (AlCl3) will dissociate into __4___(how many) ions and will cause a (greater/smaller) change boiling point than MgCl2. 19. In the solvation of solids, solubility rate increases with ( ...
... 17. (Ionic/molecular) compounds cause greater change of boiling/freezing points in solutions. 18. Aluminum chloride (AlCl3) will dissociate into __4___(how many) ions and will cause a (greater/smaller) change boiling point than MgCl2. 19. In the solvation of solids, solubility rate increases with ( ...