Biol 1406 notes Ch 2 8thed - Chemistry
... o There are 92 naturally occurring elements. o Each element has a unique symbol, usually the first one or two letters of its name. Some symbols are derived from Latin or German names. A compound is a substance that consists of two or more elements in a fixed ratio. o Table salt (sodium chloride or ...
... o There are 92 naturally occurring elements. o Each element has a unique symbol, usually the first one or two letters of its name. Some symbols are derived from Latin or German names. A compound is a substance that consists of two or more elements in a fixed ratio. o Table salt (sodium chloride or ...
General Chemistry Unit 11
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
Chemistry 1st Semester Practice Exam
... B. All atoms of a given element are identical to each other and different from those of other elements. ...
... B. All atoms of a given element are identical to each other and different from those of other elements. ...
Test - Regents
... Answer all questions in this part. Directions (66–84): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. Naturally occurring ...
... Answer all questions in this part. Directions (66–84): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 and 67 on the information below. Naturally occurring ...
Practice Questions - Elevate Education
... 4.Draw the structural formulae for formaldehyde and for formic acid. 5. Give the correct IUPAC name for formaldehyde. 6.Consider the reaction of methanol with ethanoic acid. 7. Write the balanced chemical equation to represent this reaction. 8. Draw the structural formula of the ester produced in th ...
... 4.Draw the structural formulae for formaldehyde and for formic acid. 5. Give the correct IUPAC name for formaldehyde. 6.Consider the reaction of methanol with ethanoic acid. 7. Write the balanced chemical equation to represent this reaction. 8. Draw the structural formula of the ester produced in th ...
1 Indentifying Unknown #M20 via Infrared Spectroscopy, Mass
... de calculated and will shed light on the number of double and/or triple bonds and the possibility of a ringed structure. Once the molecular formula is determined based on the information presented in both the IR and mass spectra, the 13C NMR contributes by determining symmetry and carbon bonding env ...
... de calculated and will shed light on the number of double and/or triple bonds and the possibility of a ringed structure. Once the molecular formula is determined based on the information presented in both the IR and mass spectra, the 13C NMR contributes by determining symmetry and carbon bonding env ...
Slajd 1 - Uniwersytet Warszawski
... Verification of the hypothesis was done in two ways 1) using the quantum chemical method by which thermodynamic functions, enthalpy, and free enthalpy were calculated, 2) using MS method applying ‘soft’ ionization techniques APCI and ESI, which allow samples to be analysed in a liquid mobile phase, ...
... Verification of the hypothesis was done in two ways 1) using the quantum chemical method by which thermodynamic functions, enthalpy, and free enthalpy were calculated, 2) using MS method applying ‘soft’ ionization techniques APCI and ESI, which allow samples to be analysed in a liquid mobile phase, ...
ACP Chemistry Semester 1 Final Exam - Doc-U-Ment
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
Thermochemistry 2 Matching Match each item with the correct
... Thermochemistry 2 Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
... Thermochemistry 2 Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
Honors Chapter 2
... has a definite composition – All samples of an identical substance have the identical physical and chemical properties Physical property – quality or condition of a substance that can be observed or measured without changing the substance’s composition ...
... has a definite composition – All samples of an identical substance have the identical physical and chemical properties Physical property – quality or condition of a substance that can be observed or measured without changing the substance’s composition ...
Structural Characterisation by ESI-MS of Feruloylated Arabino-oligosaccharides Synthesised by Chemoenzymatic Esterification
... FAEs have been used in the synthesis of sugar ferulates such as 5-O-(trans-feruloyl)-L-arabinofuranose [5] or O-[5-O-(trans-feruloyl)-α-L-arabinofuranosyl]-(1→5)-L-arabinofuranose [6]. More specifically, StFaeC catalyzed the transfer of the feruloyl group to L-arabinose and L-arabinobiose in a terna ...
... FAEs have been used in the synthesis of sugar ferulates such as 5-O-(trans-feruloyl)-L-arabinofuranose [5] or O-[5-O-(trans-feruloyl)-α-L-arabinofuranosyl]-(1→5)-L-arabinofuranose [6]. More specifically, StFaeC catalyzed the transfer of the feruloyl group to L-arabinose and L-arabinobiose in a terna ...
Document
... Molecular equation: balanced chemical equation showing neutral formulas for all reactants and products. Ca(NO3)2(aq) + Na2SO4(aq) → CaSO4(s) + 2 NaNO3(aq) Complete ionic equation: all strong electrolytes are written as separate ions with their own coefficients and ...
... Molecular equation: balanced chemical equation showing neutral formulas for all reactants and products. Ca(NO3)2(aq) + Na2SO4(aq) → CaSO4(s) + 2 NaNO3(aq) Complete ionic equation: all strong electrolytes are written as separate ions with their own coefficients and ...
as a PDF
... compound (left-hand axis; n estimated value; ` experimental value); (c) the standard enthalpy change of reaction 6 (^ right-hand axis). Data are from Refs. [11, 13, 14, 18 and 19]. ...
... compound (left-hand axis; n estimated value; ` experimental value); (c) the standard enthalpy change of reaction 6 (^ right-hand axis). Data are from Refs. [11, 13, 14, 18 and 19]. ...
225 Unit 7, Lab 1 - Pope John Paul II High School
... atoms and molecules, keep in mind that we never talk about a single atom (or molecule) when we use chemical equations. This is because single atoms (and molecules) are so tiny that they are difficult to isolate. Chemical equations are discussed in relation to the number of moles of reactants and pro ...
... atoms and molecules, keep in mind that we never talk about a single atom (or molecule) when we use chemical equations. This is because single atoms (and molecules) are so tiny that they are difficult to isolate. Chemical equations are discussed in relation to the number of moles of reactants and pro ...
Chapter 16: Early earth and the origin of life
... • Some prokaryotes can withstand harsh conditions – By forming endospores Internal Organization • Some prokaryotic cells – Have specialized membranes that perform metabolic functions 16.11 Prokaryotes obtain nourishment in a variety of ways • As a group – Prokaryotes exhibit much more nutritional di ...
... • Some prokaryotes can withstand harsh conditions – By forming endospores Internal Organization • Some prokaryotic cells – Have specialized membranes that perform metabolic functions 16.11 Prokaryotes obtain nourishment in a variety of ways • As a group – Prokaryotes exhibit much more nutritional di ...
The Periodic Table of Elements and Atoms…
... number of electrons as there are protons. •Protons are positively charged particles found in the nucleus. There are an equal number of protons as there are electrons. ...
... number of electrons as there are protons. •Protons are positively charged particles found in the nucleus. There are an equal number of protons as there are electrons. ...
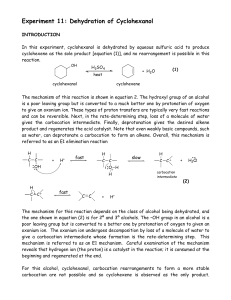
dipole/induced-dipole and dipole/induced
... Important lessons for the future: electron-withdrawing groups are not the only carbon but also decreasing around the carbon and reduce the electron density of the carbon!!! ...
... Important lessons for the future: electron-withdrawing groups are not the only carbon but also decreasing around the carbon and reduce the electron density of the carbon!!! ...