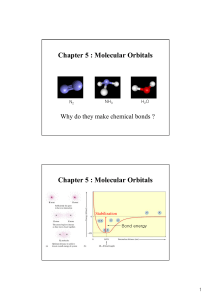
Document
... • They add to each other by opening up their carbon to carbon double bonds. • This process is called addition ...
... • They add to each other by opening up their carbon to carbon double bonds. • This process is called addition ...
uncorrected page proofs
... of all known chemicals. They include not only those compounds that were part of or were derived from plants and animals, but also all carbon compounds except for those mentioned above. Of all the elements in the periodic table, carbon is the only one that has properties that make it possible for liv ...
... of all known chemicals. They include not only those compounds that were part of or were derived from plants and animals, but also all carbon compounds except for those mentioned above. Of all the elements in the periodic table, carbon is the only one that has properties that make it possible for liv ...
Microsoft Word - Open Access Repository of Indian Theses
... The conjugate addition (1,4-addition or Michael addition) of nucleophiles to α,βunsaturated compounds is one of the most important new bond-forming strategies in synthetic organic chemistry. Aza-Michael addition is one of the important reactions especially for the synthesis of C-N heterocycles conta ...
... The conjugate addition (1,4-addition or Michael addition) of nucleophiles to α,βunsaturated compounds is one of the most important new bond-forming strategies in synthetic organic chemistry. Aza-Michael addition is one of the important reactions especially for the synthesis of C-N heterocycles conta ...
Angewandte - School of Physics
... spectra of small gold clusters, and in charge transfer from the support to the clusters, 3) impurity-doping effects that allow modification and control of the electronic structure, and consequently the chemical reactivity, of small supported clusters, through incorporation of judiciously chosen impu ...
... spectra of small gold clusters, and in charge transfer from the support to the clusters, 3) impurity-doping effects that allow modification and control of the electronic structure, and consequently the chemical reactivity, of small supported clusters, through incorporation of judiciously chosen impu ...
Solutions. Electrolytic dissociation
... OH, COOH and SO3Na groups. When wanting to synthesize a watersoluble drug, these groups are usually introduced. Water is a slightly ionic compound and dissolves metallic salts, e.g. NaCl, and ionic compounds. Cell materials are either water-soluble or water-insoluble. The smaller building blocks of ...
... OH, COOH and SO3Na groups. When wanting to synthesize a watersoluble drug, these groups are usually introduced. Water is a slightly ionic compound and dissolves metallic salts, e.g. NaCl, and ionic compounds. Cell materials are either water-soluble or water-insoluble. The smaller building blocks of ...
Hydrogenation of fatty acid methyl ester to fatty alcohol
... and pressure, and hydrogenation reactions were performed in the batch experimental system. products were analyzed by a GC equipped with a flame ionization detector (FID). 1-octanol was used as the internal standard compound for analysis. All components were identified by Micromass GCTTM GC-mass spec ...
... and pressure, and hydrogenation reactions were performed in the batch experimental system. products were analyzed by a GC equipped with a flame ionization detector (FID). 1-octanol was used as the internal standard compound for analysis. All components were identified by Micromass GCTTM GC-mass spec ...
Organolithium reagent
... completion if the acidic compound is 2 pKA units stronger than the lithium species, although in practice a larger pKA difference is required for useful rates of deprotonation of weakly acidic C-H acids. As alkyl groups are weakly electron donating, the basicity of the organolithium compound increase ...
... completion if the acidic compound is 2 pKA units stronger than the lithium species, although in practice a larger pKA difference is required for useful rates of deprotonation of weakly acidic C-H acids. As alkyl groups are weakly electron donating, the basicity of the organolithium compound increase ...
Page 1 of 25
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
... c. Definite volume; shape of container; no intermolecular attractions d. Volume and shape of container; no intermolecular attractions e. Volume and shape of container; strong intermolecular attractions 102. Which transformation is evaporation? a. liquid ---> solid d. solid ---> gas b. liquid ---> ga ...
Reacciones redox
... The second way to deliver H2 is to add two protons and two electrons to a substrat- that is, ...
... The second way to deliver H2 is to add two protons and two electrons to a substrat- that is, ...
File
... reaction is referred to as nucleophilic acylsubstitution. But the reaction is not a direct substitution. Instead, it occurs in two steps: (1) nucleophilic addition, followed by (2) elimination. ...
... reaction is referred to as nucleophilic acylsubstitution. But the reaction is not a direct substitution. Instead, it occurs in two steps: (1) nucleophilic addition, followed by (2) elimination. ...
Chapter 15: Kinetics
... changes as they are used up. The rate at any particular moment is called the instantaneous rate. It can be calculated from a concentration versus time plot. ...
... changes as they are used up. The rate at any particular moment is called the instantaneous rate. It can be calculated from a concentration versus time plot. ...
Chapter 1 Chemistry and Measurement
... Aluminum powder burns in oxygen to produce a substance called aluminum oxide. A sample of 2.00 grams of aluminum is burned in oxygen and produces 3.78 grams of aluminum oxide. How many grams of oxygen were used in this reaction? aluminum + oxygen = aluminum oxide 2.00 g + oxygen = 3.78 g oxygen = 1. ...
... Aluminum powder burns in oxygen to produce a substance called aluminum oxide. A sample of 2.00 grams of aluminum is burned in oxygen and produces 3.78 grams of aluminum oxide. How many grams of oxygen were used in this reaction? aluminum + oxygen = aluminum oxide 2.00 g + oxygen = 3.78 g oxygen = 1. ...
Nucleophilic Addition: The Grignard reagent
... To the reaction flask that contains the product from day one, add 6.0 mL of 6 M hydrochloric acid dropwise. Use a spatula to break up the solid and cap and shake the vial to help dissolve the solid. Because the left over magnesium will react with the acid to form hydrogen, and the neutralization rea ...
... To the reaction flask that contains the product from day one, add 6.0 mL of 6 M hydrochloric acid dropwise. Use a spatula to break up the solid and cap and shake the vial to help dissolve the solid. Because the left over magnesium will react with the acid to form hydrogen, and the neutralization rea ...
Brochure BITSAT-2011
... his/her answers among the 150 questions. If a candidate answers all the 150 questions (without skipping any question), the candidate will have an option of attempting 12 (twelve) extra questions, if there is still time left. These extra questions will be from Physics, Chemistry, and Mathematics only ...
... his/her answers among the 150 questions. If a candidate answers all the 150 questions (without skipping any question), the candidate will have an option of attempting 12 (twelve) extra questions, if there is still time left. These extra questions will be from Physics, Chemistry, and Mathematics only ...
Scheme of work and lesson plan booklet
... The Scheme of Work and sample Lesson plans provide examples of how to teach this unit and the teaching hours are suggestions only. Some or all of it may be applicable to your teaching. The Specification is the document on which assessment is based and specifies what content and skills need to be co ...
... The Scheme of Work and sample Lesson plans provide examples of how to teach this unit and the teaching hours are suggestions only. Some or all of it may be applicable to your teaching. The Specification is the document on which assessment is based and specifies what content and skills need to be co ...
Organic chemistry
... of all known chemicals. They include not only those compounds that were part of or were derived from plants and animals, but also all carbon compounds except for those mentioned above. Of all the elements in the periodic table, carbon is the only one that has properties that make it possible for liv ...
... of all known chemicals. They include not only those compounds that were part of or were derived from plants and animals, but also all carbon compounds except for those mentioned above. Of all the elements in the periodic table, carbon is the only one that has properties that make it possible for liv ...