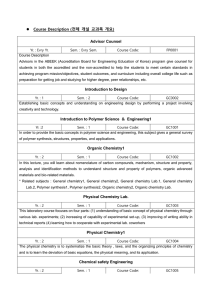
cape chemistry unit ii module i
... around the ring. That makes the ring much more reactive than it is in benzene itself. It also helps to make the -OH group's hydrogen a lot more acidic than it is in alcohols. The -OH group attached to the benzene ring in phenol has the effect of making the ring much more reactive than it would other ...
... around the ring. That makes the ring much more reactive than it is in benzene itself. It also helps to make the -OH group's hydrogen a lot more acidic than it is in alcohols. The -OH group attached to the benzene ring in phenol has the effect of making the ring much more reactive than it would other ...
organic powerpoint
... Br, I) in place of a hydrogen Name the halogen first (fluoro, chloro, bromo, iodo) then name the alkane If there are more than 2 carbons in the chain, use a number to indicate which carbon the –X group is attached to. (Number from the direction that gives the smallest number) ...
... Br, I) in place of a hydrogen Name the halogen first (fluoro, chloro, bromo, iodo) then name the alkane If there are more than 2 carbons in the chain, use a number to indicate which carbon the –X group is attached to. (Number from the direction that gives the smallest number) ...
chm238f02.exam2
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. ...
... (c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement. ...
organic problems - St. Olaf College
... 34 A C6H10 hydrocarbon forms an insoluble silver salt when treated with silver nitrate in ethanolic ammonia. Acid catalyzed hydration with a HgSO4 catalyst generates a single C6H12O ketone, and pemanganate oxidation yields a C5H10O2 carboxylic acid This compound is most likely which of the following ...
... 34 A C6H10 hydrocarbon forms an insoluble silver salt when treated with silver nitrate in ethanolic ammonia. Acid catalyzed hydration with a HgSO4 catalyst generates a single C6H12O ketone, and pemanganate oxidation yields a C5H10O2 carboxylic acid This compound is most likely which of the following ...
R,S Configurations
... usually achiral. 1 asymmetric C atom* in the compound: chiral. >1 asymmetric C atoms in the compound: may or may not be chiral. *The asymmetric atom can be an element other than C. ...
... usually achiral. 1 asymmetric C atom* in the compound: chiral. >1 asymmetric C atoms in the compound: may or may not be chiral. *The asymmetric atom can be an element other than C. ...
Chapter 9
... in the production of many important chemicals, such as aspirin, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine, which is represented by the following equation. ...
... in the production of many important chemicals, such as aspirin, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine, which is represented by the following equation. ...
Section 1 Describing Chemical Reactions Chapter 8
... Single-Displacement Reactions, continued Displacement of Halogens • Fluorine is the most-active halogen. • It can replace any of the other halogens in their compounds. • In Group 17 each element can replace any element below it, but not any element above it. Cl2(g) + 2KBr(aq) F2(g) + 2NaCl(aq) Br2(l ...
... Single-Displacement Reactions, continued Displacement of Halogens • Fluorine is the most-active halogen. • It can replace any of the other halogens in their compounds. • In Group 17 each element can replace any element below it, but not any element above it. Cl2(g) + 2KBr(aq) F2(g) + 2NaCl(aq) Br2(l ...
Section 6.3 Balancing Chemical Equations
... when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. Return to TOC ...
... when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. Return to TOC ...
Sahand University of Technology
... 1. It must wet the surfaces, that is it must spread and make a contact angle approaching zero. Intimate contact is required between the molecules of the adhesive and the atoms and molecules in the surface. When applied the adhesive will be a liquid of relatively low viscosity. 2. The adhesive must t ...
... 1. It must wet the surfaces, that is it must spread and make a contact angle approaching zero. Intimate contact is required between the molecules of the adhesive and the atoms and molecules in the surface. When applied the adhesive will be a liquid of relatively low viscosity. 2. The adhesive must t ...
Energetics 5
... during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electr ...
... during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electr ...
Exam - Vcaa
... • Write your student number in the space provided above on this page. • Check that your name and student number as printed on your answer sheet for multiple-choice questions are correct, and sign your name in the space provided to verify this. • All written responses must be in English. At the end o ...
... • Write your student number in the space provided above on this page. • Check that your name and student number as printed on your answer sheet for multiple-choice questions are correct, and sign your name in the space provided to verify this. • All written responses must be in English. At the end o ...
Booklet Chapter 3
... b. Identify which of two atoms in a polar covalent bond has a partial negative charge and which atom has a partial positive charge. c. Identify which of two atoms in an ionic bond has a negative charge and which atom has a positive charge. d. Given two bonds, determine which of the bonds would be ex ...
... b. Identify which of two atoms in a polar covalent bond has a partial negative charge and which atom has a partial positive charge. c. Identify which of two atoms in an ionic bond has a negative charge and which atom has a positive charge. d. Given two bonds, determine which of the bonds would be ex ...
SED122 - National Open University of Nigeria
... Matter is electrical in nature. Evidences for this assertion came from results of experiments of early scientists like Faraday, Thompson and Millikan. The negatively charged particle in matter is the electron, It has negligible mass. The proton is the positively charged particle. It carries the same ...
... Matter is electrical in nature. Evidences for this assertion came from results of experiments of early scientists like Faraday, Thompson and Millikan. The negatively charged particle in matter is the electron, It has negligible mass. The proton is the positively charged particle. It carries the same ...
Addition Reactions of Carbonyls Part 1
... n-Butyllithium and t-butyllithium are often used to remove hydrogen atoms you really wouldn’t consider to be particularly acidic – such as the hydrogen atoms on a benzene ring! Not all that surprising when you consider that the pKa values for their conjugate acids are ~50!!! ...
... n-Butyllithium and t-butyllithium are often used to remove hydrogen atoms you really wouldn’t consider to be particularly acidic – such as the hydrogen atoms on a benzene ring! Not all that surprising when you consider that the pKa values for their conjugate acids are ~50!!! ...
Journal Club - Clinical Chemistry
... Results: Performance of the Sandwich Assay. Table 2. Performance Characteristics of the Tacrolimus Sandwich Assay. a WBP1-3 = whole blood pool from transplant patients. b WPB4= whole blood pool from nontransplant patients spiked with tacrolimus powder. c Five whole blood samples from transplant pat ...
... Results: Performance of the Sandwich Assay. Table 2. Performance Characteristics of the Tacrolimus Sandwich Assay. a WBP1-3 = whole blood pool from transplant patients. b WPB4= whole blood pool from nontransplant patients spiked with tacrolimus powder. c Five whole blood samples from transplant pat ...
Kinetics of Oxidation of Benzyl Alcohol with Dilute Nitric Acid
... Benzaldehyde (BzH) and substituted benzaldehydes are important perfumery and pharmaceutical intermediates. Benzaldehyde is a starting material for odorants and flavors. Substituted benzaldehydes are used in the manufacture of pharmaceuticals (e.g., 2-chlorobenzaldehyde is used in the manufacture of ...
... Benzaldehyde (BzH) and substituted benzaldehydes are important perfumery and pharmaceutical intermediates. Benzaldehyde is a starting material for odorants and flavors. Substituted benzaldehydes are used in the manufacture of pharmaceuticals (e.g., 2-chlorobenzaldehyde is used in the manufacture of ...
Summer Assignment: Some Review / Basic Prep
... E) Physical properties vs. Chemical properties 1) Physical properties can be observed without changing the identity and composition of the substance e.gphyscial : odor, density, melting point, hardness, color, boiling point a) Thus, a physical change is a change in appearance, but not in the composi ...
... E) Physical properties vs. Chemical properties 1) Physical properties can be observed without changing the identity and composition of the substance e.gphyscial : odor, density, melting point, hardness, color, boiling point a) Thus, a physical change is a change in appearance, but not in the composi ...
10. Alkyl Halides - faculty at Chemeketa
... The selectivity of chlorine radical is 1.0 : 3.5 : 5.0 for 1°, 2° and 3° hydrogens, respectively. Assuming that only monochlorides are produced in the radical chain chlorination of 2,3-dimethybutane, what would be the expected ratio of the two isomeric alkyl chlorides formed in the reaction? ...
... The selectivity of chlorine radical is 1.0 : 3.5 : 5.0 for 1°, 2° and 3° hydrogens, respectively. Assuming that only monochlorides are produced in the radical chain chlorination of 2,3-dimethybutane, what would be the expected ratio of the two isomeric alkyl chlorides formed in the reaction? ...
16 Chemical Equilibrium Chapter Outline Rates of Reaction
... Chemical Equilibrium: a dynamic state in which two opposing processes (forward and reverse reactions) occur simultaneously at the same rate. ...
... Chemical Equilibrium: a dynamic state in which two opposing processes (forward and reverse reactions) occur simultaneously at the same rate. ...