Aluminum and Copper
... Possible signs of a chemical reaction include color changes, temperature changes, formation of a precipitate, and gas evolution. The reaction of aluminum and copper(II) chloride is very vigorous—the reaction mixture gets very hot as heat is released, the blue color due to the Cu(II) ions fades, t ...
... Possible signs of a chemical reaction include color changes, temperature changes, formation of a precipitate, and gas evolution. The reaction of aluminum and copper(II) chloride is very vigorous—the reaction mixture gets very hot as heat is released, the blue color due to the Cu(II) ions fades, t ...
Stoichiometry, Lab Basics, Reactions
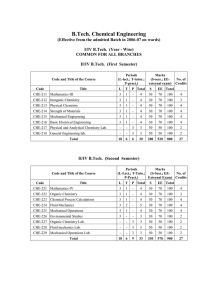
... CaCl2. What is the minimum number of moles of AgNO3 that must be added to the solution in order to precipitate all of the Cl- as AgCl (s)? (Assume all AgCl is insoluble.) A) 0.10 mol B) 0.20 mol C) 0.30 mol D) 0.40 mol E) 0.60 mol ____ 21. A 40.0 mL sample of 0.25 M KOH is added to 60.0 mL of 0.15 M ...
... CaCl2. What is the minimum number of moles of AgNO3 that must be added to the solution in order to precipitate all of the Cl- as AgCl (s)? (Assume all AgCl is insoluble.) A) 0.10 mol B) 0.20 mol C) 0.30 mol D) 0.40 mol E) 0.60 mol ____ 21. A 40.0 mL sample of 0.25 M KOH is added to 60.0 mL of 0.15 M ...
13C NMR Spectroscopy (#1d)
... DEPT (Distortionless Enhancement by Polarization Transfer), we can regain the coupling information that is lost in proton-decoupled spectra. Usually, three spectra are obtained: 1. A proton-decoupled spectrum that shows all carbon environments in the molecule; 2. A DEPT-90 spectrum showing only CH p ...
... DEPT (Distortionless Enhancement by Polarization Transfer), we can regain the coupling information that is lost in proton-decoupled spectra. Usually, three spectra are obtained: 1. A proton-decoupled spectrum that shows all carbon environments in the molecule; 2. A DEPT-90 spectrum showing only CH p ...
Chapter 2: Mass Relations in Formulas, Chemical Reactions, and
... Hence, the simplest formula or the empirical formula is CHCI. The empirical formula mass is 12 + 1 + 35.5 = 48.5 g/mol ...
... Hence, the simplest formula or the empirical formula is CHCI. The empirical formula mass is 12 + 1 + 35.5 = 48.5 g/mol ...
Organic Chemistry
... Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO3-) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen or nitrogen Carbon joins other in chains or rings and can have branches coming off of these chains or rings One molecular formula can rep ...
... Chemistry of compounds that contain carbon (except: CO, CO 2, HCN, CO3-) Carbon is covalently bonded to another carbon, hydrogen and possibly to oxygen, a halogen or nitrogen Carbon joins other in chains or rings and can have branches coming off of these chains or rings One molecular formula can rep ...
1 FORMATION OF THE ATOMIC THEORY
... requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his atomic theory, it attracted some attention. However, it failed to gain unanimous support. Some sup ...
... requires the process in which two or more atoms combine to form matter. This is the reason why Dalton’s atom is called the “chemical atom”. (c) Proof that atoms exist When Dalton initially proposed his atomic theory, it attracted some attention. However, it failed to gain unanimous support. Some sup ...
32. The reaction described by this equilibrium is
... another. In linked questions, the answer from one question is used to complete the next question. If you answer the first question incorrectly but use that answer correctly to answer the second question, you will still receive full marks for the second question The examination is 2.5 hours in length ...
... another. In linked questions, the answer from one question is used to complete the next question. If you answer the first question incorrectly but use that answer correctly to answer the second question, you will still receive full marks for the second question The examination is 2.5 hours in length ...
Carbohydrates: Occurrence, Structures and Chemistry
... group (C-5 in glucose) has the same configuration as the asymmetric center in D-glyceraldehyde and, likewise, all L-sugars are configurationally derived from L-glyceraldehyde. A convenient way to show configurational relationships was introduced by EMIL FISCHER in 1891 [11, 12], now termed Fischer p ...
... group (C-5 in glucose) has the same configuration as the asymmetric center in D-glyceraldehyde and, likewise, all L-sugars are configurationally derived from L-glyceraldehyde. A convenient way to show configurational relationships was introduced by EMIL FISCHER in 1891 [11, 12], now termed Fischer p ...
Organic Chemistry - City University of New York
... between a resonance hybrid in which the electrons are delocalized and the most stable one of its hypothetical contributing structures in which electrons are localized on particular atoms and in particular bonds. • One way to estimate the resonance energy of benzene is to compare the heats of hydro ...
... between a resonance hybrid in which the electrons are delocalized and the most stable one of its hypothetical contributing structures in which electrons are localized on particular atoms and in particular bonds. • One way to estimate the resonance energy of benzene is to compare the heats of hydro ...
Chem 314 Preorganic Evaluation
... E2 Reactions are emphasized in this section special features: biomolecular kinetics (Rate = kE2[RX][B-], single step concerted reaction, competing reaction is SN2 favored reactivity: 3oRX > 2o RX > 1oRX (none at CH3X, need Cβ-H), 1oRX will produce mainly SN2 product excet for mostly E2 with the ster ...
... E2 Reactions are emphasized in this section special features: biomolecular kinetics (Rate = kE2[RX][B-], single step concerted reaction, competing reaction is SN2 favored reactivity: 3oRX > 2o RX > 1oRX (none at CH3X, need Cβ-H), 1oRX will produce mainly SN2 product excet for mostly E2 with the ster ...
Chemical Reactions
... Predict the products using the type of reaction as a model Balance the equation ...
... Predict the products using the type of reaction as a model Balance the equation ...
CHAPTER 3 STOICHIOMETRY:
... 2) The coefficients must be the lowest whole-numbers possible. 3) Do not change or add subscripts (number of atoms of each element in a molecule). 4) Balance H and O last. 5) If a polyatomic ion stays together on both sides of the arrow, then keep it together when balancing. 2HCl + Na2CO3 → 2NaCl + ...
... 2) The coefficients must be the lowest whole-numbers possible. 3) Do not change or add subscripts (number of atoms of each element in a molecule). 4) Balance H and O last. 5) If a polyatomic ion stays together on both sides of the arrow, then keep it together when balancing. 2HCl + Na2CO3 → 2NaCl + ...
Application Note 7: Observing Spin Systems using COSY
... systems are shown in different colours, and coupling is where the lines intersect on a peak off the diagonal (crosspeak). The protons in line with this cross-peak couple to each other. Note that the methylene protons occur at different chemical shifts despite being attached to the same carbon atom, ...
... systems are shown in different colours, and coupling is where the lines intersect on a peak off the diagonal (crosspeak). The protons in line with this cross-peak couple to each other. Note that the methylene protons occur at different chemical shifts despite being attached to the same carbon atom, ...
7.1 Equilibrium PPT equilibrium1
... The equilibrium constant is a measure of the amount of products at equilibrium compared with the amount of ...
... The equilibrium constant is a measure of the amount of products at equilibrium compared with the amount of ...
Chem 331 Biochemistry
... learn the structures of selected saccharides. I think it important to know what forces stabilize the complex sugars and some of the chemistry. Much of the stereochemistry is a repeat of what you’ve already learned in organic chemistry so much of this is review and isn’t a key part of what we will go ...
... learn the structures of selected saccharides. I think it important to know what forces stabilize the complex sugars and some of the chemistry. Much of the stereochemistry is a repeat of what you’ve already learned in organic chemistry so much of this is review and isn’t a key part of what we will go ...
Mole
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
picture_as_pdf Released Materials 2013 (16551
... Much of the lead used for batteries and ammunition during the First World War and the Second World War came from galena, PbS(s). The following equations represent the reactions that are involved in refining galena to produce solid lead. Equation I Equation II Equation III ...
... Much of the lead used for batteries and ammunition during the First World War and the Second World War came from galena, PbS(s). The following equations represent the reactions that are involved in refining galena to produce solid lead. Equation I Equation II Equation III ...