Some uses of mischmetall in organic synthesis
... terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We th ...
... terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We th ...
WORD VERSION See HERE for missing picture
... ____ 13. Which phrase best describes the effect of a catalyst on a chemical reaction? a. increases the temperature c. decreases the reaction rate b. increases the volume of reactants d. decreases the activation energy ____ 14. Enzymes affect chemical reactions in living organisms by a. changing the ...
... ____ 13. Which phrase best describes the effect of a catalyst on a chemical reaction? a. increases the temperature c. decreases the reaction rate b. increases the volume of reactants d. decreases the activation energy ____ 14. Enzymes affect chemical reactions in living organisms by a. changing the ...
... Reactions of enaminone 1 with some selected primary and secondary amines were carried out to obtain new enaminones of biological interest. Thus, reacting 1 with piperidine and/or morpholine, in boiling dioxane, led to 4-[(1-piperidyl)/(4-morpholinyl)]methylenepyrazolidinediones 12a and 12b, in 9598% ...
13-Elimination Reactions
... competing elimination reaction takes place under some conditions instead of the intended substitution reaction. In a β-elimination reaction, the substrate loses two atoms or two groups of atoms to form a π bond. This new double bond forms after, or concurrent with, the loss of the leaving groups. Us ...
... competing elimination reaction takes place under some conditions instead of the intended substitution reaction. In a β-elimination reaction, the substrate loses two atoms or two groups of atoms to form a π bond. This new double bond forms after, or concurrent with, the loss of the leaving groups. Us ...
Chapter 12 Oxidation-Reduction Reactions
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
Chemistry FIFTH EDITION by Steven S. Zumdahl University of Illinois
... Step 2: NO3 + CO (g) NO2 (g) + CO2 (g) Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
... Step 2: NO3 + CO (g) NO2 (g) + CO2 (g) Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
Amines By
... Primary amines can be named by replacing the e with the word amine. The prefix can also be amino. amine can also be named by naming the parent group followed by a space and the word amine. Ex: propane = propanamine, aminopropane, or propyl amine. Secondary amines can be named by replacing the ...
... Primary amines can be named by replacing the e with the word amine. The prefix can also be amino. amine can also be named by naming the parent group followed by a space and the word amine. Ex: propane = propanamine, aminopropane, or propyl amine. Secondary amines can be named by replacing the ...
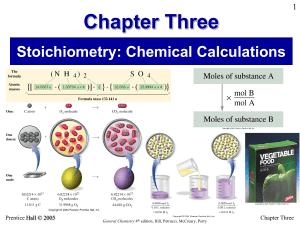
Chapter Three - CNG Chemistry
... In these examples, note the common practice of expressing molar mass and Avogadro’s number with at least one significant figure more than the number of significant figures in the least precisely known quantity. Doing this ensures that the precision of the calculated results is limited only by the le ...
... In these examples, note the common practice of expressing molar mass and Avogadro’s number with at least one significant figure more than the number of significant figures in the least precisely known quantity. Doing this ensures that the precision of the calculated results is limited only by the le ...
The adiabatic flame temperature
... Use of global reactions to express chemistry does not provide a basis for understanding what is actually happening. For example, the overall reaction equation for hydrogen combustion: ...
... Use of global reactions to express chemistry does not provide a basis for understanding what is actually happening. For example, the overall reaction equation for hydrogen combustion: ...
Chapter 17: Reaction Energy and Reaction Kinetics
... Modifying the chemical equation to show the amount of energy produced during the reaction gives the following expression. 2H2(g) + O2(g) → 2H2O(g) + 483.6 kJ This expression is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat dur ...
... Modifying the chemical equation to show the amount of energy produced during the reaction gives the following expression. 2H2(g) + O2(g) → 2H2O(g) + 483.6 kJ This expression is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat dur ...
Chemistry FIFTH EDITION by Steven S. Zumdahl University of Illinois
... Heterogeneous Catalysts -- most often involves gaseous reactants being adsorbed on the surface of a solid catalyst. EXAMPLE: Hydrogenation of ethylene ...
... Heterogeneous Catalysts -- most often involves gaseous reactants being adsorbed on the surface of a solid catalyst. EXAMPLE: Hydrogenation of ethylene ...
Free Radical Chemistry and the Preparation of Alkyl
... in carbon-skeleton building substitution reactions (SN2 mechanism, Ch. 11) Alkyl halides and organic oxidation and reduction (10.9): Formation of Grignard or Gilman reagents can be considered an organic reduction since the electron density on the carbon atom increases. Reductions increase the e- den ...
... in carbon-skeleton building substitution reactions (SN2 mechanism, Ch. 11) Alkyl halides and organic oxidation and reduction (10.9): Formation of Grignard or Gilman reagents can be considered an organic reduction since the electron density on the carbon atom increases. Reductions increase the e- den ...
UNSYMMETRICAL DINUCLEAR RHODIUM COMPLEXES WITH
... arsanylarylthiolates AsS–, AsS22– and AsS33– is less well developed. Although a number of examples of transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligan ...
... arsanylarylthiolates AsS–, AsS22– and AsS33– is less well developed. Although a number of examples of transition metal complexes of triorganoarsines which are efficient catalysts in organic synthesis are already known, the combination of an arsenic atom and one or more sulfur atoms in the same ligan ...
Physical Properties of Macromolecules Glass Transitions in Amorphous Polymers
... Inorganic models of transition-metal coordination between d-block salts and functional polymers are introduced and analyzed to explain increases in Tg via coordination crosslinks. d-Electron configurations and stabilization of metal d-electrons due to the surrounding ligands in the first-shell coord ...
... Inorganic models of transition-metal coordination between d-block salts and functional polymers are introduced and analyzed to explain increases in Tg via coordination crosslinks. d-Electron configurations and stabilization of metal d-electrons due to the surrounding ligands in the first-shell coord ...
amines amide - TangHua2012-2013
... •Some amines are beneficial to body, but some are really harmful, •Amines are used to make amine drugs (mimic or interfere neurotransmitters) ...
... •Some amines are beneficial to body, but some are really harmful, •Amines are used to make amine drugs (mimic or interfere neurotransmitters) ...
Chapter 24. Amines
... • The N lone-pair electrons in arylamines are delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
... • The N lone-pair electrons in arylamines are delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
Chapter 24. Amines
... • The N lone-pair electrons in arylamines are delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
... • The N lone-pair electrons in arylamines are delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
chemistry -- questions -
... __ 70. The characteristic way in which atoms of an element react is most related to the a) number of electrons in the outermost shell. b) number of electrons in the innermost shell. c) number of neutrons in the nucleus. d) size of the nucleus. __ 71. Which of the following statements is NOT true abo ...
... __ 70. The characteristic way in which atoms of an element react is most related to the a) number of electrons in the outermost shell. b) number of electrons in the innermost shell. c) number of neutrons in the nucleus. d) size of the nucleus. __ 71. Which of the following statements is NOT true abo ...
History of Organic Chemistry
... There are about 2 times as many inorganic compounds as organic compounds d. There are about 2 times as many organic compounds as organic compounds There are thousands of times more organic than inorganic compounds. The enormous number of carbon compounds is believed to be due, in large part, to the ...
... There are about 2 times as many inorganic compounds as organic compounds d. There are about 2 times as many organic compounds as organic compounds There are thousands of times more organic than inorganic compounds. The enormous number of carbon compounds is believed to be due, in large part, to the ...