Which is Aromatic?
... 2. The p-orbital on each carbon atom overlaps above and below the σ-framework with the p-orbital on the adjacent carbon atom ...
... 2. The p-orbital on each carbon atom overlaps above and below the σ-framework with the p-orbital on the adjacent carbon atom ...
Photosynthesis in Hydrogen-Dominated Atmospheres
... water, if they have a defined surface at all (Seager and Rogers in preparation). However rocky planets with a thin, hydrogen-dominated atmosphere can have a surface temperature compatible with liquid water. H2:H2 collision-induced absorption (CIA) of near-infrared (NIR) light provides a strong green ...
... water, if they have a defined surface at all (Seager and Rogers in preparation). However rocky planets with a thin, hydrogen-dominated atmosphere can have a surface temperature compatible with liquid water. H2:H2 collision-induced absorption (CIA) of near-infrared (NIR) light provides a strong green ...
ISOMERISM - Knockhardy
... the greater the degree of branching the lower the boiling point branching decreases the effectiveness of intermolecular attractive forces less energy has to be put in to separate the molecules boiling points can also vary between isomers containing different functional groups e.g alcohols and ethers ...
... the greater the degree of branching the lower the boiling point branching decreases the effectiveness of intermolecular attractive forces less energy has to be put in to separate the molecules boiling points can also vary between isomers containing different functional groups e.g alcohols and ethers ...
Chapter 20 Thermodynamics
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
... is the same even though they took very different paths to get to the mountain top. This is the power of all the state functions in thermodynamics---we only need to now the start and the finish bulk parameters. ...
[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION
... 2)Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3)Balanced Chemical equations – The chemical equation in which the no. of atoms of different elements is same on both sides of the arrow is calle ...
... 2)Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3)Balanced Chemical equations – The chemical equation in which the no. of atoms of different elements is same on both sides of the arrow is calle ...
Organic Chemistry – Summary of Reactions and Conditions
... Heat under reflux with NaOH, then neutralise with HCl (can also be hydrolysed with an acid catalyst) CH3CH2CN + OH- (aq) + H2O (l) → CH3CH2COO- (aq) + NH3 (aq) CH3CH2COO- (aq) + HCl (aq) → CH3CH2COOH (aq) + Cl- (aq) ...
... Heat under reflux with NaOH, then neutralise with HCl (can also be hydrolysed with an acid catalyst) CH3CH2CN + OH- (aq) + H2O (l) → CH3CH2COO- (aq) + NH3 (aq) CH3CH2COO- (aq) + HCl (aq) → CH3CH2COOH (aq) + Cl- (aq) ...
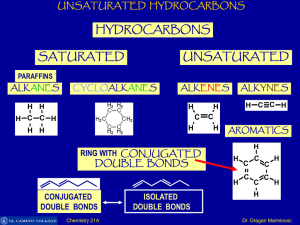
Unsaturated Hydrocarbons
... C-atom overlap with two sp2-hybrid orbital of two other C-atoms to form sigma bonds. In this way there are six sigma bonds are formed between six C-atoms which are 120o apart. Remaining six sp2-orbital of six C-atoms overlap with 1s orbital of six H-atoms individually to form six sigma bonds. Since ...
... C-atom overlap with two sp2-hybrid orbital of two other C-atoms to form sigma bonds. In this way there are six sigma bonds are formed between six C-atoms which are 120o apart. Remaining six sp2-orbital of six C-atoms overlap with 1s orbital of six H-atoms individually to form six sigma bonds. Since ...
When Gold Is Not Noble: Nanoscale Gold
... The catalytic activity (measured via the amount of desorbed carbon dioxide) on Au8 (0.4% ML) clusters deposited on defectpoor MgO (100) films observed during one heating cycle (Figure 1b), reveals a very small combustion of CO at temperatures between 150 and 250 K. Surprisingly, CO combustion on Au8 ...
... The catalytic activity (measured via the amount of desorbed carbon dioxide) on Au8 (0.4% ML) clusters deposited on defectpoor MgO (100) films observed during one heating cycle (Figure 1b), reveals a very small combustion of CO at temperatures between 150 and 250 K. Surprisingly, CO combustion on Au8 ...
The Role of Medicinal Chemistry in Canadian Pharmacy
... 2735 B.C. – Babylonian, Chinese, Indian cultures 400 B.C. – Greek culture (ancient apothecary) ...
... 2735 B.C. – Babylonian, Chinese, Indian cultures 400 B.C. – Greek culture (ancient apothecary) ...
structure and substitution. Thus, the culties associated with
... mass and pmr spectra are very useful in elucidations of the structure near a functional group. dues are present, hoviever ...
... mass and pmr spectra are very useful in elucidations of the structure near a functional group. dues are present, hoviever ...
chemistry intermediate may 2010 marking scheme
... (iii) a reagent that can be used to carry out the second stage. Aqueous NaOH (2) (6 marks) (d) Name the reagent needed to change sodium propanoate into ethane and write an equation for the reaction that takes place. Soda lime (2) CH3CH2COONa + NaOH = CH3CH3 + Na2CO3 (2) (4 marks) (Total 16 marks) 17 ...
... (iii) a reagent that can be used to carry out the second stage. Aqueous NaOH (2) (6 marks) (d) Name the reagent needed to change sodium propanoate into ethane and write an equation for the reaction that takes place. Soda lime (2) CH3CH2COONa + NaOH = CH3CH3 + Na2CO3 (2) (4 marks) (Total 16 marks) 17 ...
Ruthenium(II) Complexes Bearing a Pyridyl-Supported Pyrazolyl
... ppm, respectively. These results suggest that all the imidazolyl, pyrazolyl, and carbonyl moieties in complex 7 are coordinated to the metal center. The 1H NMR signals of the corresponding pyrazolyl, imidalzolyl, and pyridyl CH groups in complex 8 were shifted upfield about 0.1 ppm, and the 13C NMR ...
... ppm, respectively. These results suggest that all the imidazolyl, pyrazolyl, and carbonyl moieties in complex 7 are coordinated to the metal center. The 1H NMR signals of the corresponding pyrazolyl, imidalzolyl, and pyridyl CH groups in complex 8 were shifted upfield about 0.1 ppm, and the 13C NMR ...
Experiment 1 - Melting Points - NAU jan.ucc.nau.edu web server
... a physical property that can be used for its identification. It is a measure of the amount of kinetic energy (heat) that must be supplied to the particles of the substance in order to overcome the intermolecular forces (such as Van der Waals, dipole-dipole, and Hbonding) that confine them to the sol ...
... a physical property that can be used for its identification. It is a measure of the amount of kinetic energy (heat) that must be supplied to the particles of the substance in order to overcome the intermolecular forces (such as Van der Waals, dipole-dipole, and Hbonding) that confine them to the sol ...
1012_4th Exam_1020619 - NTOU-Chem
... D) Ethylene glycol forms a film on the surface of the water to prevent ice formation in the radiator. E) Ethylene glycol will remain in the radiator after the water evaporates. This prevents freezing in cold climates. Answer: A ...
... D) Ethylene glycol forms a film on the surface of the water to prevent ice formation in the radiator. E) Ethylene glycol will remain in the radiator after the water evaporates. This prevents freezing in cold climates. Answer: A ...
Chapter 19 Chemical Thermodynamics
... Analyze: We are given four equations and asked to predict the sign of ΔS for each chemical reaction. Plan: The sign of ΔS will be positive if there is an increase in temperature, an increase in the volume in which the molecules move, or an increase in the number of gas particles in the reaction. The ...
... Analyze: We are given four equations and asked to predict the sign of ΔS for each chemical reaction. Plan: The sign of ΔS will be positive if there is an increase in temperature, an increase in the volume in which the molecules move, or an increase in the number of gas particles in the reaction. The ...
ANALYSIS OF THE SILVER GROUP CATIONS
... separate the mixture into subgroups that consist of just a few ions. Then it may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test ...
... separate the mixture into subgroups that consist of just a few ions. Then it may be possible to test for one particular ion in the presence of just one or two others. Alternatively, each subgroup of just a few ions may be separated further so that each ion in the subgroup ends up in a different test ...
Microwave-Assisted Esterification of N -Acetyl-L-Phenylalanine Using Modified Mukaiyama s Reagents: A New Approach Involving Ionic Liquids
... the isolated ester; 4 TBA = tributylamine; 5 TEA = triethylamine; 6 MIM = 1methylimidazole; 7 DCM = dichloromethane (extra care should be practiced when performing microwave experiments in DCM!). Many organic solvents have been investigated in the Mukaiyama’s esterification, which include dichlorome ...
... the isolated ester; 4 TBA = tributylamine; 5 TEA = triethylamine; 6 MIM = 1methylimidazole; 7 DCM = dichloromethane (extra care should be practiced when performing microwave experiments in DCM!). Many organic solvents have been investigated in the Mukaiyama’s esterification, which include dichlorome ...
Amine - presentation
... Boiling points increase with molecular mass Amines have higher boiling points than corresponding alkanes because of their intermolecular hydrogen bonding Quarternary ammonium salts are ionic and exist as salts ...
... Boiling points increase with molecular mass Amines have higher boiling points than corresponding alkanes because of their intermolecular hydrogen bonding Quarternary ammonium salts are ionic and exist as salts ...
The 2016 AP Chemistry Exam will be Monday
... passed since you have had a chemistry course, it is imperative that you come to class the first day with Sections A and B completed. This summer assignment is not optional, and completing the assignment in a thorough and focused manner will contribute to a student’s success in this course and on the ...
... passed since you have had a chemistry course, it is imperative that you come to class the first day with Sections A and B completed. This summer assignment is not optional, and completing the assignment in a thorough and focused manner will contribute to a student’s success in this course and on the ...
full text pdf
... of functional monomer, non-covalent bonds or stronger covalent bonds are formed. Their formation is determined by a number of factors which will ultimately determine the quality and durability of the obtained polymer [Luliński et al. 2008]. The most important parameters that influence the molecular ...
... of functional monomer, non-covalent bonds or stronger covalent bonds are formed. Their formation is determined by a number of factors which will ultimately determine the quality and durability of the obtained polymer [Luliński et al. 2008]. The most important parameters that influence the molecular ...




![[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION](http://s1.studyres.com/store/data/008930072_1-5a35e1ae8e3204ea88999f1418a93013-300x300.png)