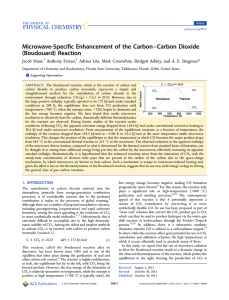
An Overview of Carbonyl Compound Chemistry
... as the final products. For example, when organometallic compounds, Grignard or organolithium reagents, are used as the carbanion species, they can react with the ketone or aldehyde intermediates very rapidly to form a second tetrahedral intermediate, in which no good leaving groups are present. Over ...
... as the final products. For example, when organometallic compounds, Grignard or organolithium reagents, are used as the carbanion species, they can react with the ketone or aldehyde intermediates very rapidly to form a second tetrahedral intermediate, in which no good leaving groups are present. Over ...
Synthesis of Novel Steroid-Peptoid Hybrid Macrocycles by
... design of macrocyclic hosts wherein conformational freedom can be kept under close control [1-4]. The cholane skeleton of bile acids is very suitable for receptor design as its curved geometry allows positioning of multiple binding functionalities directed towards the concave side. Different types o ...
... design of macrocyclic hosts wherein conformational freedom can be kept under close control [1-4]. The cholane skeleton of bile acids is very suitable for receptor design as its curved geometry allows positioning of multiple binding functionalities directed towards the concave side. Different types o ...
Organic Handout
... Problem number 1 Lots of carbon and hydrogen atoms Pain to draw them all implicit hydrogens (on the chalk board) ...
... Problem number 1 Lots of carbon and hydrogen atoms Pain to draw them all implicit hydrogens (on the chalk board) ...
Ch 4 Student
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
... • Limiting Reactant – reactant that is completely consumed and limits amount of product • Reactant in excess – reactant present in greater quantity than limiting reactant • Theoretical Yield – amount of product made based on consumption of all the limiting reactant • Actual Yield – amount of product ...
CP - Supplemental Activities
... of!the!particle!ever!be!zero!inside!the!box?!Why!or!why!not?!! Can!the!energy!of!the!particle!be!any!value!inside!a!given!box?!Why!or!why!not?! 4. Using!the!equation!for!the!energy!of!a!particle!in!a!one!dimensional!box,!please!describe!the! affect!the!changing!the!length!of!box!would!have!on!the!en ...
... of!the!particle!ever!be!zero!inside!the!box?!Why!or!why!not?!! Can!the!energy!of!the!particle!be!any!value!inside!a!given!box?!Why!or!why!not?! 4. Using!the!equation!for!the!energy!of!a!particle!in!a!one!dimensional!box,!please!describe!the! affect!the!changing!the!length!of!box!would!have!on!the!en ...
Unit 3 Exam Level Questions
... B the equilibrium position will move to the right C the concentration of Cu2+(aq) ions will increase D the equilibrium position will not be affected. 9. Steam and carbon monoxide react to form an equilibrium mixture. CO(g) + H2O(g) H2(g) + CO2(g) Which of the following graphs shows how the rates of ...
... B the equilibrium position will move to the right C the concentration of Cu2+(aq) ions will increase D the equilibrium position will not be affected. 9. Steam and carbon monoxide react to form an equilibrium mixture. CO(g) + H2O(g) H2(g) + CO2(g) Which of the following graphs shows how the rates of ...
2nd Nine Weeks Notes
... a. A plot of 1/[A] vs. t will produce a straight line with a slope equal to k. b. [A] depends on time and can be used to calculate [A] at any time t, provided k and [A]o are known. 4. Half-Life. * Equation: ...
... a. A plot of 1/[A] vs. t will produce a straight line with a slope equal to k. b. [A] depends on time and can be used to calculate [A] at any time t, provided k and [A]o are known. 4. Half-Life. * Equation: ...
CIS Exam Questions
... B the equilibrium position will move to the right C the concentration of Cu2+(aq) ions will increase D the equilibrium position will not be affected. 9. Steam and carbon monoxide react to form an equilibrium mixture. CO(g) + H2O(g) H2(g) + CO2(g) Which of the following graphs shows how the rates of ...
... B the equilibrium position will move to the right C the concentration of Cu2+(aq) ions will increase D the equilibrium position will not be affected. 9. Steam and carbon monoxide react to form an equilibrium mixture. CO(g) + H2O(g) H2(g) + CO2(g) Which of the following graphs shows how the rates of ...
Grignard Reagents
... synthesis by reasoning backward from the the target molecule to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ulti ...
... synthesis by reasoning backward from the the target molecule to a starting compound using known and reliable reactions. “it is a problem solving technique for transforming the structure of a synthetic target molecule (TM) to a sequence of progressively simpler structures along the pathway which ulti ...
Chemistry Standard Level Chapter 1
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
synthesis-structure relationship in the aqueous ethylene glycol
... shows a remarkable stability, due to the very strong hydrogen bonds between adjacent layers, therefore it is practically insoluble in water and in common organic solvents. In pure state, its composition does not alter with time and it can only be destructurated in a strongly acidic medium or by trea ...
... shows a remarkable stability, due to the very strong hydrogen bonds between adjacent layers, therefore it is practically insoluble in water and in common organic solvents. In pure state, its composition does not alter with time and it can only be destructurated in a strongly acidic medium or by trea ...
1 Quantitative chemistry - Pearson Schools and FE Colleges
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
... reactions involve changes in smell, colour and texture and these are difficult to quantify. Lavoisier appreciated the importance of attaching numbers to properties and recognized the need for precise measurement. His use of the balance allowed changes in mass to be used to analyse chemical reactions. ...
Classifying organic materials by oxygen-to
... part of the low-volatility fraction of the oxidation products of volatile organic compounds, is one important subclass of atmospheric organic compounds and can be highly CCN active (Hartz et al., 2005; VanReken et al., 2005; King et al., 2007; Wex et al., 2009; Kuwata et al., 2011). Both laboratory ...
... part of the low-volatility fraction of the oxidation products of volatile organic compounds, is one important subclass of atmospheric organic compounds and can be highly CCN active (Hartz et al., 2005; VanReken et al., 2005; King et al., 2007; Wex et al., 2009; Kuwata et al., 2011). Both laboratory ...
Defined megadalton hyaluronan polymer standards. Anal
... biotin/avidin technology. The new dendritic-like molecules approximate linear HA chains based on electrophoretic migration, and thus the complexes are useful as standards for gels. ...
... biotin/avidin technology. The new dendritic-like molecules approximate linear HA chains based on electrophoretic migration, and thus the complexes are useful as standards for gels. ...
Oxygen Isotope Effects as Structural and Mechanistic Probes in
... a resurgence of interest in heavy-atom isotope effect measurements, however, because of their utility in comparing reactions of enzymes to those of structurally defined, biomimetic compounds. The measurements also complement theoretical calculations of structure and mechanism. Such combined experime ...
... a resurgence of interest in heavy-atom isotope effect measurements, however, because of their utility in comparing reactions of enzymes to those of structurally defined, biomimetic compounds. The measurements also complement theoretical calculations of structure and mechanism. Such combined experime ...
Discussion Questions
... a. The limiting reactant is the one with the higher molar mass. b. A is the limiting reactant because you need 6 moles of A and have 4 moles. c. B is the limiting reactant because you have fewer moles of B than A. d. B is the limiting reactant because three A molecules react with each B mole ...
... a. The limiting reactant is the one with the higher molar mass. b. A is the limiting reactant because you need 6 moles of A and have 4 moles. c. B is the limiting reactant because you have fewer moles of B than A. d. B is the limiting reactant because three A molecules react with each B mole ...
Week 7 - Acid-base, redox
... With titration, one combines two reactants to reach a stoichiometric proportion or endpoint. The endpoint is often visualized by adding an indicator. At the endpoint, one can analyze an analyte (moles, grams, percentage, or concentration). Titration Works for any reaction type. The calculations for ...
... With titration, one combines two reactants to reach a stoichiometric proportion or endpoint. The endpoint is often visualized by adding an indicator. At the endpoint, one can analyze an analyte (moles, grams, percentage, or concentration). Titration Works for any reaction type. The calculations for ...