Week 7 - Acid-base, redox
... With titration, one combines two reactants to reach a stoichiometric proportion or endpoint. The endpoint is often visualized by adding an indicator. At the endpoint, one can analyze an analyte (moles, grams, percentage, or concentration). Titration Works for any reaction type. The calculations for ...
... With titration, one combines two reactants to reach a stoichiometric proportion or endpoint. The endpoint is often visualized by adding an indicator. At the endpoint, one can analyze an analyte (moles, grams, percentage, or concentration). Titration Works for any reaction type. The calculations for ...
Chemical Reactions and Equations - 2012 Book Archive
... two solid rocket boosters, which use a solid fuel mixture that contains mainly ammonium perchlorate and powdered aluminum. The chemical reaction between these substances produces aluminum oxide, water, nitrogen gas, and hydrogen chloride. Although the solid rocket boosters each have a significantly ...
... two solid rocket boosters, which use a solid fuel mixture that contains mainly ammonium perchlorate and powdered aluminum. The chemical reaction between these substances produces aluminum oxide, water, nitrogen gas, and hydrogen chloride. Although the solid rocket boosters each have a significantly ...
Organic - UCLA Chemistry and Biochemistry
... the characteristic apparent quartet (J = 12 Hz) for Ha that is also seen in the spectrum of Id.' Likewise the stereochemistry of 9b was determined primarily from its highfield 'H NMR spectrum which showed the expected coupling constants for the conformation drawn, namely: Ha dd, J = 11.8, 6.8 Hz;Hb ...
... the characteristic apparent quartet (J = 12 Hz) for Ha that is also seen in the spectrum of Id.' Likewise the stereochemistry of 9b was determined primarily from its highfield 'H NMR spectrum which showed the expected coupling constants for the conformation drawn, namely: Ha dd, J = 11.8, 6.8 Hz;Hb ...
Sample chapter - Pharmaceutical Press
... In sp2 hybridisation the 2s and two of the 2p orbitals are combined to create three sp2 hybrid orbitals. The remaining 2p orbital remains unchanged by the hybridisation process. Each hybrid orbital has a 1/3 contribution from each of the three starting atomic orbitals. The three sp2 hybrid orbitals ...
... In sp2 hybridisation the 2s and two of the 2p orbitals are combined to create three sp2 hybrid orbitals. The remaining 2p orbital remains unchanged by the hybridisation process. Each hybrid orbital has a 1/3 contribution from each of the three starting atomic orbitals. The three sp2 hybrid orbitals ...
Section 2 Types of Chemical Reactions
... Single-Displacement Reactions, continued Displacement of Halogens • Fluorine is the most-active halogen. • It can replace any of the other halogens in their compounds. • In Group 17 each element can replace any element below it, but not any element above it. Cl2(g) + 2KBr(aq) F2(g) + 2NaCl(aq) Br2(l ...
... Single-Displacement Reactions, continued Displacement of Halogens • Fluorine is the most-active halogen. • It can replace any of the other halogens in their compounds. • In Group 17 each element can replace any element below it, but not any element above it. Cl2(g) + 2KBr(aq) F2(g) + 2NaCl(aq) Br2(l ...
Document
... – H2, Cl2, Br2, HCl, HBr is added to an unsaturated hyrdrocarbon. Both atoms are added to where the double (or triple) bond was ...
... – H2, Cl2, Br2, HCl, HBr is added to an unsaturated hyrdrocarbon. Both atoms are added to where the double (or triple) bond was ...
to Dowload Part 1: PowerPoint Presentation.
... STOP! Learning Check #8 2. What is the more common name of the aldehyde: methanal? Draw it’s structure. 3. What is a common use of aldehydes in the food industry? ...
... STOP! Learning Check #8 2. What is the more common name of the aldehyde: methanal? Draw it’s structure. 3. What is a common use of aldehydes in the food industry? ...
Chemical Equilibrium Equilibrium A state where the reactants and
... Knowing the equilibrium constant allows us to predict several important features of the reaction. 1) the tendency of the reaction to ___________ (but not the _______________) 2) whether a given set of concentrations represent an __________________ condition 3) the equilibrium position that will be ...
... Knowing the equilibrium constant allows us to predict several important features of the reaction. 1) the tendency of the reaction to ___________ (but not the _______________) 2) whether a given set of concentrations represent an __________________ condition 3) the equilibrium position that will be ...
The first practical method for asymmetric epoxidation
... The procedure described above for epoxidation of geraniol calls for 1 equiv of both titanium isopropoxide and diethyl tartrate. This is by no means necessary in all cases. With reactive allylic alcohols (la, 2a, 3a, and 4a in Table I), a catalytic amount (e.g., 0.1 equiv) of both Ti(O-i-Pr)4 and die ...
... The procedure described above for epoxidation of geraniol calls for 1 equiv of both titanium isopropoxide and diethyl tartrate. This is by no means necessary in all cases. With reactive allylic alcohols (la, 2a, 3a, and 4a in Table I), a catalytic amount (e.g., 0.1 equiv) of both Ti(O-i-Pr)4 and die ...
Chemistry - CBSE Guess
... 16. What are enantiomers? How can they be identified? 17. What are the micro-alloys? Explain with two examples. 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the ...
... 16. What are enantiomers? How can they be identified? 17. What are the micro-alloys? Explain with two examples. 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the ...
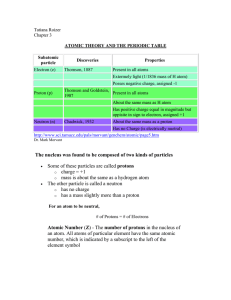
atomic theory and the periodic table
... the electron is further from the nucleus - this is an orbital at the second energy level. If you look carefully, you will notice that there is another region of slightly higher electron density (where the dots are thicker) nearer the nucleus. ("Electron density" is another way of talking about how l ...
... the electron is further from the nucleus - this is an orbital at the second energy level. If you look carefully, you will notice that there is another region of slightly higher electron density (where the dots are thicker) nearer the nucleus. ("Electron density" is another way of talking about how l ...
Stockholm University
... the transient allylboronates with aldehyde and imine electrophiles. In a typical reaction the diboronate 1, the allylacetate 2, the appropriate electrophile (3 or 4) and catalytic amounts of Pd2(dba)3 [dba = (dibenzylidene)acetone] were mixed in DMSO and after the allotted reaction time (Table 1) th ...
... the transient allylboronates with aldehyde and imine electrophiles. In a typical reaction the diboronate 1, the allylacetate 2, the appropriate electrophile (3 or 4) and catalytic amounts of Pd2(dba)3 [dba = (dibenzylidene)acetone] were mixed in DMSO and after the allotted reaction time (Table 1) th ...
A Brief History of Organic Chemistry
... undermined the idea of Vitalism because an organic compound was produced from an inorganic one. However, it also represented the discovery of isomerism - the possibility of two or more different structures (ammonium cyanate crystals and urea crystals) based on the same chemical formula (N2H4CO). Che ...
... undermined the idea of Vitalism because an organic compound was produced from an inorganic one. However, it also represented the discovery of isomerism - the possibility of two or more different structures (ammonium cyanate crystals and urea crystals) based on the same chemical formula (N2H4CO). Che ...
Faculty of Chemistry, Brno University of Technology Purkynova 118
... The results of the processes represented in Fig. 3 were studied during two different experiments but with the same discharge conditions and set parameters. But although they were observed simultaneously in the same water solution and at the same time, the evaluation of the dye and H2 O2 concentratio ...
... The results of the processes represented in Fig. 3 were studied during two different experiments but with the same discharge conditions and set parameters. But although they were observed simultaneously in the same water solution and at the same time, the evaluation of the dye and H2 O2 concentratio ...
Chapter 4 Carbon and the Molecular Diversity of Life
... Chapter 4 Carbon and the Molecular Diversity of Life All organisms are composed mostly of chemical structures based on the element carbon. This chapter builds upon information and concepts introduced in Chapters 2 and 3 and extends the descriptions and analysis to more detailed consideration of the ...
... Chapter 4 Carbon and the Molecular Diversity of Life All organisms are composed mostly of chemical structures based on the element carbon. This chapter builds upon information and concepts introduced in Chapters 2 and 3 and extends the descriptions and analysis to more detailed consideration of the ...