SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... 1. State the meaning of relative atomic mass based on C- 12 scale 2. State why C 12 is used as a standard for determining relative atomic mass and relative molecular mass 3. Caculate the relative molecular mass of substances. A student is able to 1. Define a mole as the amount of matter that contain ...
... 1. State the meaning of relative atomic mass based on C- 12 scale 2. State why C 12 is used as a standard for determining relative atomic mass and relative molecular mass 3. Caculate the relative molecular mass of substances. A student is able to 1. Define a mole as the amount of matter that contain ...
equilibrium questions - Southington Public Schools
... (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. (i) Calculate the molar concentration of OH–(aq) in the saturated solution. (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of ...
... (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. (i) Calculate the molar concentration of OH–(aq) in the saturated solution. (ii) Write the solubility-product constant expression for M(OH)2. (iii) Calculate the value of ...
Stoichiometry: Calculations with Chemical Formulas and
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Final Study Guide (Semester 2) Answer Key
... CuSO4(aq) + 2KOH(aq) Cu(OH)2(s ) + K2SO4(aq) Which compounds above are strong electrolytes? CuSO4 , KOH , K2SO4 a. Which chemical above is not soluble in water? Cu(OH)2 c. Which chemical above is the precipitate? Cu(OH)2 2. Solutions of Barium nitrate and potassium sulfate are mixed. ***The first ...
... CuSO4(aq) + 2KOH(aq) Cu(OH)2(s ) + K2SO4(aq) Which compounds above are strong electrolytes? CuSO4 , KOH , K2SO4 a. Which chemical above is not soluble in water? Cu(OH)2 c. Which chemical above is the precipitate? Cu(OH)2 2. Solutions of Barium nitrate and potassium sulfate are mixed. ***The first ...
Homework Solutions Week 6
... volume of permanganate needed is 2/5 of the volume of oxalic acid. i.e. 108.0 mL x 2/5 = 43.0 mL For the second part of the question Volume of oxalic acid needed is = 5/2 volume of MnO4- = 108.0 x 5/2 = 270.0 mL 7-11. Limestone consists mainly of the mineral calcite, CaCO3. The carbonate content of ...
... volume of permanganate needed is 2/5 of the volume of oxalic acid. i.e. 108.0 mL x 2/5 = 43.0 mL For the second part of the question Volume of oxalic acid needed is = 5/2 volume of MnO4- = 108.0 x 5/2 = 270.0 mL 7-11. Limestone consists mainly of the mineral calcite, CaCO3. The carbonate content of ...
Discussion Questions
... 62. A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M HNO3 solution. If the unknown base concentration is 0.0300 M, give some possible identities for the unknown base. 63. A student had 1.00 L of a 1.00 M acid solution. Much to the surprise of ...
... 62. A 30.0-mL sample of an unknown strong base is neutralized after the addition of 12.0 mL of a 0.150 M HNO3 solution. If the unknown base concentration is 0.0300 M, give some possible identities for the unknown base. 63. A student had 1.00 L of a 1.00 M acid solution. Much to the surprise of ...
Chapter 8 - Cengage Learning
... Would you say ammonia (NH3) is mostly nitrogen or mostly hydrogen? Your answer depends on if you are looking at the number of atoms or the mass of the atoms. In terms of numbers of atoms, ammonia is ¾ hydrogen (there are four atoms making up an ammonia molecule, and three of them are hydrogen). But ...
... Would you say ammonia (NH3) is mostly nitrogen or mostly hydrogen? Your answer depends on if you are looking at the number of atoms or the mass of the atoms. In terms of numbers of atoms, ammonia is ¾ hydrogen (there are four atoms making up an ammonia molecule, and three of them are hydrogen). But ...
Chemical Bonding
... Explaining the Properties of Ionic Compounds Ionic compounds have similar properties: They are solids at SATP with high melting points, and they are electrolytes. We can hypothesize that these properties might be the result of the bonds formed between the ions, holding them firmly in a rigid structu ...
... Explaining the Properties of Ionic Compounds Ionic compounds have similar properties: They are solids at SATP with high melting points, and they are electrolytes. We can hypothesize that these properties might be the result of the bonds formed between the ions, holding them firmly in a rigid structu ...
____ 1. The energy required to convert a ground
... 16. The safest and most effective emergency procedure to treat an acid splash on skin is to do which of the following immediately? a. Dry the affected area with paper towels d. Flush the affected area with water and then with a dilute NaHCO3 solution b. Sprinkle the affected area with powdered e. Fl ...
... 16. The safest and most effective emergency procedure to treat an acid splash on skin is to do which of the following immediately? a. Dry the affected area with paper towels d. Flush the affected area with water and then with a dilute NaHCO3 solution b. Sprinkle the affected area with powdered e. Fl ...
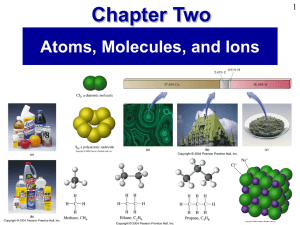
Prentice Hall Ch 02 Atoms Molecules Ions
... Strategy We usually write mass ratios in a form such as “the ratio of O to Mg is 0.6583:1.” The first number represents the mass of the first element named—in this case, a mass of oxygen, say 0.6583 g oxygen—and the second number represents the mass of the second element named—here a mass of magnesi ...
... Strategy We usually write mass ratios in a form such as “the ratio of O to Mg is 0.6583:1.” The first number represents the mass of the first element named—in this case, a mass of oxygen, say 0.6583 g oxygen—and the second number represents the mass of the second element named—here a mass of magnesi ...