answers to part a of the national high school
... The fuel indicated in the triangle could be any flammable substance, not just gasoline or oil etc. It could be wood or paper or absolutely anything that can burn. And heat doesnt need to be a flame it may be a spark, or simply something that is very hot such as an engine part or exhaust pipe ...
... The fuel indicated in the triangle could be any flammable substance, not just gasoline or oil etc. It could be wood or paper or absolutely anything that can burn. And heat doesnt need to be a flame it may be a spark, or simply something that is very hot such as an engine part or exhaust pipe ...
here - Math Berkeley
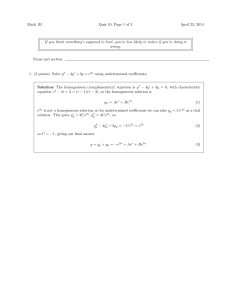
... 3. (4 points) Solve y 00 + y = sec x using variation of parameters. Solution: The homogeneous equation is y 00 + y = 0, with characteristic equation r2 + 1 = (r + i)(r − i) = 0, so for two linearly independent homogeneous solutions we can take y1 = cos x, y2 = sin x. Variation of parameters tells us ...
... 3. (4 points) Solve y 00 + y = sec x using variation of parameters. Solution: The homogeneous equation is y 00 + y = 0, with characteristic equation r2 + 1 = (r + i)(r − i) = 0, so for two linearly independent homogeneous solutions we can take y1 = cos x, y2 = sin x. Variation of parameters tells us ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
+ OH - (aq) - Miss Gerges
... Would Cu(s) be oxidized by Zn2+(aq) ions the way Zn(s) is oxidized by Cu2+(aq) ions? Cu(s) + ZnCl2 (aq) ...
... Would Cu(s) be oxidized by Zn2+(aq) ions the way Zn(s) is oxidized by Cu2+(aq) ions? Cu(s) + ZnCl2 (aq) ...
The mole and calculations
... Think about the size of this number. 109 is a billion. 1018 is a billion billion. 1023 is one hundred thousand billion billion. If you had 6.022 x 1023 dollars you could spend a billion dollars a second for your entire lifetime and still have used less than 0.001% of your money. If C atoms were ...
... Think about the size of this number. 109 is a billion. 1018 is a billion billion. 1023 is one hundred thousand billion billion. If you had 6.022 x 1023 dollars you could spend a billion dollars a second for your entire lifetime and still have used less than 0.001% of your money. If C atoms were ...
Chapter 9
... This means that joint variation occurs if we say y = k * x * z , where x and z are variables that are not zero, and k is a constant that is not zero. For example, we know Area = base * height on a rectangle. This means A = 1 * B * H. Thus we say the area of a rectangle varies jointly with the width ...
... This means that joint variation occurs if we say y = k * x * z , where x and z are variables that are not zero, and k is a constant that is not zero. For example, we know Area = base * height on a rectangle. This means A = 1 * B * H. Thus we say the area of a rectangle varies jointly with the width ...
Stoichiometry and the Mole
... If any of the ratios are not a whole number, multiply all the ratios by a factor to make it a whole number – If ratio is ?.5 then multiply by 2; if ?.33 or ?.67 then multiply by 3; if ?.25 or ?.75 then multiply by 4 ...
... If any of the ratios are not a whole number, multiply all the ratios by a factor to make it a whole number – If ratio is ?.5 then multiply by 2; if ?.33 or ?.67 then multiply by 3; if ?.25 or ?.75 then multiply by 4 ...
Empirical Formula, Molecular Formula, Percent Composition
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
Unit 6- Math of Chemistry
... vol of CO2(g) = x ratio C2H6(g) to CO2(g) = 2:4 30L/ 2 = X/4 X= 60L of CO2 2. How many moles of H2O will be produced from the complete combustion of 3 mol of C2H6 according to the equation? mol C2H6 = 3 mol mol water = x mol ratio ethane to water = 2:6 3mol C2H6/ 2 mol C2H6 = x/6mol water X=9mol wat ...
... vol of CO2(g) = x ratio C2H6(g) to CO2(g) = 2:4 30L/ 2 = X/4 X= 60L of CO2 2. How many moles of H2O will be produced from the complete combustion of 3 mol of C2H6 according to the equation? mol C2H6 = 3 mol mol water = x mol ratio ethane to water = 2:6 3mol C2H6/ 2 mol C2H6 = x/6mol water X=9mol wat ...
1 - Weebly
... using numbers more easily appreciated than in real experiments. Note: Empirical formula means the simplest whole number ratio formula found by experiment. In real laboratory experiments only a fraction of a gram or a few grams of elements would be used, and a more 'tricky' mole calculation method is ...
... using numbers more easily appreciated than in real experiments. Note: Empirical formula means the simplest whole number ratio formula found by experiment. In real laboratory experiments only a fraction of a gram or a few grams of elements would be used, and a more 'tricky' mole calculation method is ...
1st-Year-ch-wise-test
... (8) In exothermic reactions, ∆H is taken as (a) positive (b) negative (c) zero (d) none of the above (9) When no. of moles of reactants and that of products are equal , then Kc has units (a) moles/dm3 (b) moles2/dm-6 (c) moles-2/dm+6 (d) no units (10) The process of surrounding of ions by solvent mo ...
... (8) In exothermic reactions, ∆H is taken as (a) positive (b) negative (c) zero (d) none of the above (9) When no. of moles of reactants and that of products are equal , then Kc has units (a) moles/dm3 (b) moles2/dm-6 (c) moles-2/dm+6 (d) no units (10) The process of surrounding of ions by solvent mo ...
ХИМИЯ НА АНГЛИЙСКОМ ЯЗЫКЕ
... 2.1. The ratio of the mass of oxygen to carbon atom is 1.3329. What is the mass of the oxygen atom? 2.2. The ratio of the mass of bromine atom to carbon is 6.650. What is the mass of the bromine atom? 2.3. Calculate the number of moles in each of the following: a) 3.01x1022 N2 molecules; b) 4.82x102 ...
... 2.1. The ratio of the mass of oxygen to carbon atom is 1.3329. What is the mass of the oxygen atom? 2.2. The ratio of the mass of bromine atom to carbon is 6.650. What is the mass of the bromine atom? 2.3. Calculate the number of moles in each of the following: a) 3.01x1022 N2 molecules; b) 4.82x102 ...