chemistry
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
... questions in this examination. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet. Your answer sheet for Part A and Part B–1 is the ...
Stoichiometry - HCC Learning Web
... Avogadro’s Number • In a lab, we cannot work with individual molecules. They are too small. • 6.02 × 1023 atoms or molecules is an amount that brings us to lab size. It is ONE MOLE. • One mole of 12C has a mass of 12.000 g. © 2015 Pearson Education, Inc. ...
... Avogadro’s Number • In a lab, we cannot work with individual molecules. They are too small. • 6.02 × 1023 atoms or molecules is an amount that brings us to lab size. It is ONE MOLE. • One mole of 12C has a mass of 12.000 g. © 2015 Pearson Education, Inc. ...
2015 chemistry
... (iii) This conversion can also be catalysed by an enzyme. Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ___________________________ ...
... (iii) This conversion can also be catalysed by an enzyme. Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ___________________________ ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
solliqsol - chemmybear.com
... (b) Elemental analysis of this unknown compound yields the following percentages by weight H=9.74%; C=38.70%; O=51.56%. Determine the molecular formula for the compound. (c) Complete combustion of a 1.05 gram sample of the compound with the stoichiometric amount of oxygen gas produces a mixture of H ...
... (b) Elemental analysis of this unknown compound yields the following percentages by weight H=9.74%; C=38.70%; O=51.56%. Determine the molecular formula for the compound. (c) Complete combustion of a 1.05 gram sample of the compound with the stoichiometric amount of oxygen gas produces a mixture of H ...
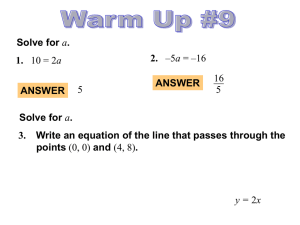
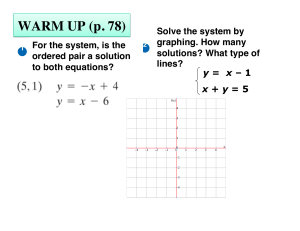
4.7.14 Write Equations Parallel and Perpendicular Notes
... • Graphs: Lines Never Intersect and are in the same plane • Equations: ...
... • Graphs: Lines Never Intersect and are in the same plane • Equations: ...
Factoring
... You can run this formula in reverse whenever you are subtracting two perfect squares. For instance, if we see x 2 25 , we recognize that both x 2 and 25 are perfect squares. We can therefore factor it as x 5x 5 . Other examples include: x 2 64 x 8x 8 ...
... You can run this formula in reverse whenever you are subtracting two perfect squares. For instance, if we see x 2 25 , we recognize that both x 2 and 25 are perfect squares. We can therefore factor it as x 5x 5 . Other examples include: x 2 64 x 8x 8 ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Expressing the concentration of solutions
... Solvent is the liquid in which the solute is dissolved an aqueous solution has water as solvent A saturated solution is one where the concentration is at a maximum - no more solute is able to dissolve. A solution is composed of a solvent which is the dissolving medium and a solute which is t ...
... Solvent is the liquid in which the solute is dissolved an aqueous solution has water as solvent A saturated solution is one where the concentration is at a maximum - no more solute is able to dissolve. A solution is composed of a solvent which is the dissolving medium and a solute which is t ...