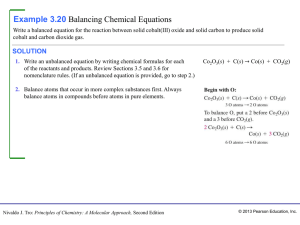
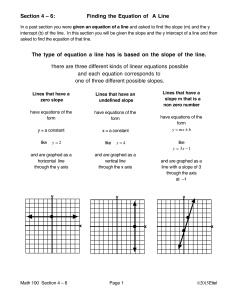
3 - LPS
... Identification. State whether each of the following is a physical or chemical change or property by writing an ”A” if it is a physical change, “B” if it is a physical property, “C” if it is a chemical change, or “D” if it is a chemical property. ...
... Identification. State whether each of the following is a physical or chemical change or property by writing an ”A” if it is a physical change, “B” if it is a physical property, “C” if it is a chemical change, or “D” if it is a chemical property. ...
CHAPTER 4: CHEMICAL QUANTITIES and AQUEOUS REACTIONS
... Therefore, if the concentration of one reactant is known, we can find out the concentration another reactant required for complete neutralization. This can be measured by ‘titration’ (with the use of a chemical indicator). The point at which the indicator changes color is called ‘end point’. 3) OXID ...
... Therefore, if the concentration of one reactant is known, we can find out the concentration another reactant required for complete neutralization. This can be measured by ‘titration’ (with the use of a chemical indicator). The point at which the indicator changes color is called ‘end point’. 3) OXID ...
Chemistry
... 1. If 495g of NaOH is dissolved to a final total volume of 20.0 L, what is the molarity of the solution? 2. How many moles of the indicated solute does each of the following solutions contain? a) 2.50 L of 13.1 M HCl b) 15.6 mL of 0.155 M NaOH 3. What mass of the indicated solute does each of the fo ...
... 1. If 495g of NaOH is dissolved to a final total volume of 20.0 L, what is the molarity of the solution? 2. How many moles of the indicated solute does each of the following solutions contain? a) 2.50 L of 13.1 M HCl b) 15.6 mL of 0.155 M NaOH 3. What mass of the indicated solute does each of the fo ...
Document
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
... SORT The problem gives the mass of carbon dioxide and asks you to find the mass of glucose that can be produced. ...
Document
... neutral compound is 0. HCl H2O K2O 5. The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. ...
... neutral compound is 0. HCl H2O K2O 5. The sum of the oxidation numbers in a polyatomic ion is the charge on the ion. ...
AP Chemistry: Total Notes Review
... o Resonance: when one Lewis structure can’t accurately describe a molecule (due to something like a double bond that “resonates” between atoms as in ozone or benzene); the electrons in the shifting bond are delocalized o Exceptions to the octet rule: 1: Odd number of electrons: rare, but you’ll just ...
... o Resonance: when one Lewis structure can’t accurately describe a molecule (due to something like a double bond that “resonates” between atoms as in ozone or benzene); the electrons in the shifting bond are delocalized o Exceptions to the octet rule: 1: Odd number of electrons: rare, but you’ll just ...
Chemical Measurements
... Moving from AMU to grams • The use of atomic mass units (amu) are impractical in the chemistry lab where the preferred unit of measurement is grams. • Scientists needed to establish a relationship between # of atoms and masses of atoms. ...
... Moving from AMU to grams • The use of atomic mass units (amu) are impractical in the chemistry lab where the preferred unit of measurement is grams. • Scientists needed to establish a relationship between # of atoms and masses of atoms. ...
Chapter
... Ionic Compounds • metals + nonmetals • no individual molecule units, instead have a 3-dimensional array of cations and anions made of formula units • many contain polyatomic ions several atoms attached together in one ion ...
... Ionic Compounds • metals + nonmetals • no individual molecule units, instead have a 3-dimensional array of cations and anions made of formula units • many contain polyatomic ions several atoms attached together in one ion ...
Electron configuration
... Camels store the fat tristearin (C57H110O6) in the hump. As well as being a source of energy, the fat is a source of water, because when it is used the reaction 2 C57H110O6(s) + 163 O2(g) 114 CO2(g) + 110 H2O(l) takes place. What mass of water can be made from 1.0 kg of fat? ...
... Camels store the fat tristearin (C57H110O6) in the hump. As well as being a source of energy, the fat is a source of water, because when it is used the reaction 2 C57H110O6(s) + 163 O2(g) 114 CO2(g) + 110 H2O(l) takes place. What mass of water can be made from 1.0 kg of fat? ...
Lab # 18
... 4. Compare and contrast the Celsius (°C) and the Kelvin (K) or absolute temperature scale. Include boiling point and melting point of water, as well as absolute zero. 5. State Charles’ Law. 6. Write Charles’ Law as a mathematical formula. 7. What is the equation used to convert between degrees Celsi ...
... 4. Compare and contrast the Celsius (°C) and the Kelvin (K) or absolute temperature scale. Include boiling point and melting point of water, as well as absolute zero. 5. State Charles’ Law. 6. Write Charles’ Law as a mathematical formula. 7. What is the equation used to convert between degrees Celsi ...
Chapter 6: Moles, Molar Mass, Percent Composition and Formulas
... ii) It’s impossible to count atoms with your hands. iii) Numbers of moles are smaller and easier to do math with than big numbers of atoms and molecules. 6) Convert moles of an atom to grams a) I need 2.0 moles of copper (Cu) for an experiment. How many grams is that? b) Atomic mass of Cu = 63.55 g/ ...
... ii) It’s impossible to count atoms with your hands. iii) Numbers of moles are smaller and easier to do math with than big numbers of atoms and molecules. 6) Convert moles of an atom to grams a) I need 2.0 moles of copper (Cu) for an experiment. How many grams is that? b) Atomic mass of Cu = 63.55 g/ ...
Example 1-2
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
... Elements in the same column have similar properties. Each column is referred to as a periodic family or group. The horizontal rows are called periods. Elements on the right side of the periodic table are nonmetals; they form anions, or negatively charged ions. Elements on the left side of the period ...
The Complete Notes - Joliet Junior College
... What are the basic building blocks of all matter, be it a diamond, a tree or the air around us? ...
... What are the basic building blocks of all matter, be it a diamond, a tree or the air around us? ...