Practice Test 3: Answer Key
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
ppt - Wits Structural Chemistry
... to its formula weight (in amu). One HCl molecule weighs 36.46 amu One mol of HCl weighs ...
... to its formula weight (in amu). One HCl molecule weighs 36.46 amu One mol of HCl weighs ...
Step 2
... Step 5 Multiply the oxidation half-reaction by 8, so that equal numbers of electrons are lost and gained. 8NO2 + 8H2O → 8NO3− + 16H+ + 8e− Step 6 Add the half reactions. 8NO2 + 8H2O → 8NO3− + 16H+ + 8e− ClO4− + 8H+ + 8e− → Cl− + 4H2O________ 8NO2 +8H2O +ClO4− +8H+ +8e−→8NO3-+16H++8e−+Cl−+4H2O Step 7 ...
... Step 5 Multiply the oxidation half-reaction by 8, so that equal numbers of electrons are lost and gained. 8NO2 + 8H2O → 8NO3− + 16H+ + 8e− Step 6 Add the half reactions. 8NO2 + 8H2O → 8NO3− + 16H+ + 8e− ClO4− + 8H+ + 8e− → Cl− + 4H2O________ 8NO2 +8H2O +ClO4− +8H+ +8e−→8NO3-+16H++8e−+Cl−+4H2O Step 7 ...
Amount of Substance
... EQUAL VOLUMES OF ALL GASES UNDER THE SAME CONDITIONS OF TEMPERATURE AND PRESSURE CONTAIN EQUAL NUMBERS OF MOLECULES. Thus it follows that one mole of any gas under a given set of temperature and pressure conditions occupies the same volume. This is called the MOLAR VOLUME. At 293K and 1 atmosphere p ...
... EQUAL VOLUMES OF ALL GASES UNDER THE SAME CONDITIONS OF TEMPERATURE AND PRESSURE CONTAIN EQUAL NUMBERS OF MOLECULES. Thus it follows that one mole of any gas under a given set of temperature and pressure conditions occupies the same volume. This is called the MOLAR VOLUME. At 293K and 1 atmosphere p ...
PowerPoint ******
... s meta and s para values indicates that they represent a measure of the electron-donating and electron-attracting powers of the substituents. (1) Strongly electron-attracting groups such as N2+, NO2, and CF3 groups have large positive s values - increase of the acidity constants of benzoic acids (2) ...
... s meta and s para values indicates that they represent a measure of the electron-donating and electron-attracting powers of the substituents. (1) Strongly electron-attracting groups such as N2+, NO2, and CF3 groups have large positive s values - increase of the acidity constants of benzoic acids (2) ...
Algebraically Finding the y-intercept with the Slope and another Point
... Algebraically Finding the y-intercept Colleen wants to know how much a chick weighs when it is hatched. Colleen tracked one of her chickens and found it grew steadily by about 5.2 grams each day since it was born. Nine days after it hatched, the chick weighed 98.4 grams. Algebraically determine how ...
... Algebraically Finding the y-intercept Colleen wants to know how much a chick weighs when it is hatched. Colleen tracked one of her chickens and found it grew steadily by about 5.2 grams each day since it was born. Nine days after it hatched, the chick weighed 98.4 grams. Algebraically determine how ...
4_ Chemical reactions
... 1. Write the names, then and formulas of reactants (unit 3 naming). 2. Exchange cations and write the names, then formulas of products (unit 3 naming) 3. Write a chemical equation to showing the formulas of reactants and products. 4. From the solubility rules include the (aq) for soluble and (s) for ...
... 1. Write the names, then and formulas of reactants (unit 3 naming). 2. Exchange cations and write the names, then formulas of products (unit 3 naming) 3. Write a chemical equation to showing the formulas of reactants and products. 4. From the solubility rules include the (aq) for soluble and (s) for ...
Experiment 22
... key principle that allows us to make a system in equilibrium behave as we wish. Consider the reaction A (aq) ⇌ B(aq) + C(aq) ...
... key principle that allows us to make a system in equilibrium behave as we wish. Consider the reaction A (aq) ⇌ B(aq) + C(aq) ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... The most basic piece of personal protective equipment is a pair of goggles, and these will always be made available to students. Like a calculator for mathematics, and running shoes for physical education goggles are personal pieces of equipment best owned by students. When students own their own go ...
... The most basic piece of personal protective equipment is a pair of goggles, and these will always be made available to students. Like a calculator for mathematics, and running shoes for physical education goggles are personal pieces of equipment best owned by students. When students own their own go ...
MOLES AND CALCULATIONS USING THE MOLE CONCEPT
... 1. A mole is the amount of any substance that contains as many elementary entities as there are atoms in exactly 1.00 g of hydrogen-1. 2. A mole is the amount ... in exactly 12.00 g of carbon-12. 3. 6.02 x 1023 of anything 4. It is important to state the entities involved: atoms, molecules, ions, el ...
... 1. A mole is the amount of any substance that contains as many elementary entities as there are atoms in exactly 1.00 g of hydrogen-1. 2. A mole is the amount ... in exactly 12.00 g of carbon-12. 3. 6.02 x 1023 of anything 4. It is important to state the entities involved: atoms, molecules, ions, el ...
Topic 1: Quantitative chemistry
... the molecular formula is known, the molecular formula can be obtained. Example: Calculate the molecular formula of a compound with a molecular mass of 84g and an empirical formula of CH2. ...
... the molecular formula is known, the molecular formula can be obtained. Example: Calculate the molecular formula of a compound with a molecular mass of 84g and an empirical formula of CH2. ...
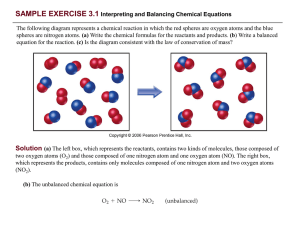
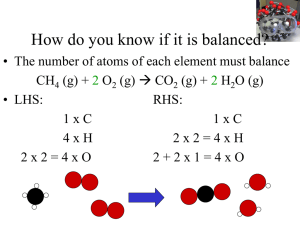
PRACTICE EXERCISE - Needham.K12.ma.us
... Finally, we check the number of atoms of each element and find that we have two Na atoms, four H atoms, and two O atoms on each side of the equation. The equation is balanced. Comment: Notice that in balancing this equation, we moved back and forth placing a coefficient in front of H2O then NaOH, an ...
... Finally, we check the number of atoms of each element and find that we have two Na atoms, four H atoms, and two O atoms on each side of the equation. The equation is balanced. Comment: Notice that in balancing this equation, we moved back and forth placing a coefficient in front of H2O then NaOH, an ...