IChO 35 Theoretical Exam
... Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quantities used temperatures as low as ...
... Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quantities used temperatures as low as ...
Unit 10: Chemical Reactions
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
... The substances that undergo a chemical reaction are the reactants. The new substances formed are the products. Special symbols are written after formulas in equations to show a substance’s state. The designations for solid, liquid, or gas, are (s), (l), and (g), respectively. A substance dissolv ...
Selective Gas-Phase Cleavage at the Peptide
... nucleophilic attack on a protonated carbonyl28-31 via a fivemembered ring, see Scheme 1.) The approach in the present study is to use a fixed-charge chemical group to “mimic” a protonated Arg residue (i.e., an Arg residue that presumably sequesters an ionizing proton). This “mimic” consists of a φ3P ...
... nucleophilic attack on a protonated carbonyl28-31 via a fivemembered ring, see Scheme 1.) The approach in the present study is to use a fixed-charge chemical group to “mimic” a protonated Arg residue (i.e., an Arg residue that presumably sequesters an ionizing proton). This “mimic” consists of a φ3P ...
Core_Class_Science_Chemistry_for_the_web 838.3 KB
... Independent variables are changed in an experiment Dependent variables change in response to the independent variable. A theory is a hypothesis that is supported by many experiments. Scientific methods can be use in pure research or in applied research. Some scientific discoveries are accidental, an ...
... Independent variables are changed in an experiment Dependent variables change in response to the independent variable. A theory is a hypothesis that is supported by many experiments. Scientific methods can be use in pure research or in applied research. Some scientific discoveries are accidental, an ...
Solubility and Solubility Equilibrium
... First things first, you have to memorize the basic solubility rules in order to (1) know which salts dissociate (break apart) in solution and (2) which ions combine to form precipitates when you mix solutions. The other part of this is that if you are given the name of a compound, you have to know t ...
... First things first, you have to memorize the basic solubility rules in order to (1) know which salts dissociate (break apart) in solution and (2) which ions combine to form precipitates when you mix solutions. The other part of this is that if you are given the name of a compound, you have to know t ...
Review #7: Solutions, Acids and Bases 1. Definitions: a) Solution: a
... example, bromothymol blue turns yellow in an acid, green in a neutral and blue in a basic solution. Phenolphthalein is colourless in an acidic or neutral solution, but pink in a base. Litmus is red in an acid and blue in a base. 2. Compare the chemical and physical properties of acids and bases: ...
... example, bromothymol blue turns yellow in an acid, green in a neutral and blue in a basic solution. Phenolphthalein is colourless in an acidic or neutral solution, but pink in a base. Litmus is red in an acid and blue in a base. 2. Compare the chemical and physical properties of acids and bases: ...
Questions - Scheikundeolympiade
... Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quantities used temperatures as low as ...
... Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quantities used temperatures as low as ...
First Semester Final Review
... a. It first melts to a liquid and then boils at about 70 oC. b. It first melts to a liquid and then boils at about 30oC. c. It melts to a liquid at a temperature of about 20oC and remains a liquid until the temperature is greater than 100oC. d. It sublimes to a vapor at an equilibrium temperature of ...
... a. It first melts to a liquid and then boils at about 70 oC. b. It first melts to a liquid and then boils at about 30oC. c. It melts to a liquid at a temperature of about 20oC and remains a liquid until the temperature is greater than 100oC. d. It sublimes to a vapor at an equilibrium temperature of ...
Chemical Reactions
... mole is the amount of substance that contains as many atoms, molecules, or ions as there are atoms in exactly 12 g of carbon-12. The important point here is that whether we are dealing with a mole of iron atoms, a mole of methane molecules, or a mole of sodium ions, a mole always contains the same n ...
... mole is the amount of substance that contains as many atoms, molecules, or ions as there are atoms in exactly 12 g of carbon-12. The important point here is that whether we are dealing with a mole of iron atoms, a mole of methane molecules, or a mole of sodium ions, a mole always contains the same n ...
PDF (chapter_8)
... and the data acquisition system used in this investigation were based on designs previously described by Hill and co-workers17,29 and have been described in detail by Johnson et al.14. The drift length of the ion mobility spectrometer was 13.65 cm and was operated in the positive mode. A drift volta ...
... and the data acquisition system used in this investigation were based on designs previously described by Hill and co-workers17,29 and have been described in detail by Johnson et al.14. The drift length of the ion mobility spectrometer was 13.65 cm and was operated in the positive mode. A drift volta ...
Chapter 12 Oxidation-Reduction Reactions
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
... The operational definition of oxidation is any reaction involving molecular oxygen. The theoretical definition of oxidation is the loss of electrons. The operational definition of reduction is the extracting of metals from metal ore. The theoretical definition of reduction is the gain of electrons. ...
1 - A-Level Chemistry
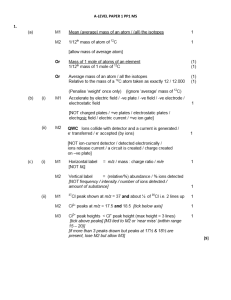
... Do not allow ‘to get an accurate reading’ or ‘reading drifts’ on its own. Allow ‘temperature variations affect readings’. ...
... Do not allow ‘to get an accurate reading’ or ‘reading drifts’ on its own. Allow ‘temperature variations affect readings’. ...