Worksheet: Acid base problems - AP level
... Example #10: If 0.50 moles Ca(OH)2 is slurried in 0.50 L deionized water and treated with 0.50 moles of CO2 gas in a closed system, the liquid phase of this system will have a pH closest to what value? Solution: After the Ca(OH)2 and the CO2 react, we are left with some calcium carbonate, an insolub ...
... Example #10: If 0.50 moles Ca(OH)2 is slurried in 0.50 L deionized water and treated with 0.50 moles of CO2 gas in a closed system, the liquid phase of this system will have a pH closest to what value? Solution: After the Ca(OH)2 and the CO2 react, we are left with some calcium carbonate, an insolub ...
NATIONAL HIGH SCHOOL CHEMISTRY EXAMINATION (1995
... 18. Formic acid (HCO2 H) is a weak monoprotic acid in aqueous solution. An aqueous solution is made by dissolving 1.00 mol of formic acid in sufficient water to make 1.00 L of solution. Which one of the following species is present in largest concentration? a) H 3 O+ ...
... 18. Formic acid (HCO2 H) is a weak monoprotic acid in aqueous solution. An aqueous solution is made by dissolving 1.00 mol of formic acid in sufficient water to make 1.00 L of solution. Which one of the following species is present in largest concentration? a) H 3 O+ ...
Equations in One Variable I
... • [3, 5) is the implied domain of the equation loge (5 − x) = x − 3. We can only take the logarithm of a number if it is positive, so 5 − x > 0, or equivalently, 5 > x. We can only take the square-root of a number if it is greater than or equal to 0, so x − 3 ≥ 0, or equivalently, x ≥ 3. The set of ...
... • [3, 5) is the implied domain of the equation loge (5 − x) = x − 3. We can only take the logarithm of a number if it is positive, so 5 − x > 0, or equivalently, 5 > x. We can only take the square-root of a number if it is greater than or equal to 0, so x − 3 ≥ 0, or equivalently, x ≥ 3. The set of ...
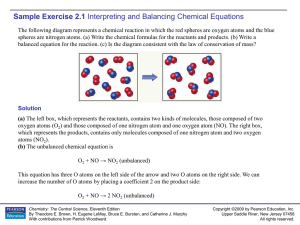
Sample Exercise 2.1
... Analyze We are given both the amount of a substance (0.350 mol) and its chemical formula (C 6H12O6). The unknown is the number of H atoms in the sample. Plan Avogadro’s number provides the conversion factor between the number of moles of C 6H12O6 and the number of molecules of C6H12O6. Once we know ...
... Analyze We are given both the amount of a substance (0.350 mol) and its chemical formula (C 6H12O6). The unknown is the number of H atoms in the sample. Plan Avogadro’s number provides the conversion factor between the number of moles of C 6H12O6 and the number of molecules of C6H12O6. Once we know ...
1 2 - Mrs. Williams` Class
... Solving Equations by 1-2 Adding or Subtracting An equation is a mathematical statement that two expressions are equal. A solution of an equation is a value of the variable that makes the equation true. To find solutions, isolate the variable. A variable is isolated when it appears by itself on one ...
... Solving Equations by 1-2 Adding or Subtracting An equation is a mathematical statement that two expressions are equal. A solution of an equation is a value of the variable that makes the equation true. To find solutions, isolate the variable. A variable is isolated when it appears by itself on one ...
Year 11 C2 Mock Exam Revision Questions
... sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of ............................................... to form phosphorus trifluoride .................................. ...
... sentences below which are about this compound. Phosphorus trifluoride is made up of phosphorus and fluorine ................................ These are joined together by sharing pairs of ............................................... to form phosphorus trifluoride .................................. ...
CHEMICAL EQUATIONS - Clayton State University
... - The coefficients in a chemical equation are the smallest set of whole numbers that balance the equation C2H5OH(l) + O2(g) → 2CO2(g) + 3H2O(g) 3(1x2)=6 H atoms (5+1)=6 H atoms ...
... - The coefficients in a chemical equation are the smallest set of whole numbers that balance the equation C2H5OH(l) + O2(g) → 2CO2(g) + 3H2O(g) 3(1x2)=6 H atoms (5+1)=6 H atoms ...
Ch 3
... A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine(N2H4) and dinitrogen tetraoxide(N2O4), which ignite on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1.00x102g of N2H4 and 2.00x102g of N2O4 are mixed? ...
... A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine(N2H4) and dinitrogen tetraoxide(N2O4), which ignite on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1.00x102g of N2H4 and 2.00x102g of N2O4 are mixed? ...
Measurements
... .3 The concentration of MgCl2 molecules is close to 0. Sometimes the molarity of a strong electrolyte is called the formal concentration (F), to emphasize that the substance is really converted to other species in solution. When we say that the “concentration” of MgCl2 is 0.054 M in seawater, we are ...
... .3 The concentration of MgCl2 molecules is close to 0. Sometimes the molarity of a strong electrolyte is called the formal concentration (F), to emphasize that the substance is really converted to other species in solution. When we say that the “concentration” of MgCl2 is 0.054 M in seawater, we are ...
Packet 1 - Kentucky Community and Technical College System
... 1. Sodium bicarbonate is also known as baking soda. If 38.9 g of it is dissolved in enough water to make 500 mL of solution, what is the Molarity of the solution? 2. If 25.3 g of sodium carbonate is dissolved in enough water to make 250 mL of solution. What is the Molar concentration of the sodium i ...
... 1. Sodium bicarbonate is also known as baking soda. If 38.9 g of it is dissolved in enough water to make 500 mL of solution, what is the Molarity of the solution? 2. If 25.3 g of sodium carbonate is dissolved in enough water to make 250 mL of solution. What is the Molar concentration of the sodium i ...