Distributed Atomic Polarizabilities of Amino Acids and their
... polarizabilities derived, using the Clausius-Mossotti equation, from experimental measurements of molar refraction in aqueous solution at ...
... polarizabilities derived, using the Clausius-Mossotti equation, from experimental measurements of molar refraction in aqueous solution at ...
Chapter 3 Molecules, Compounds, and Chemical Equations q
... - result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other generally e a y formed o ed when e metal ea a atoms o s bo bonded ded to o - ge nonmetal atoms ii.) Covalent bonds – result when two atoms share some of their electrons. – genera ...
... - result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other generally e a y formed o ed when e metal ea a atoms o s bo bonded ded to o - ge nonmetal atoms ii.) Covalent bonds – result when two atoms share some of their electrons. – genera ...
Calculation Booklet - Clydebank High School
... formed in the neutralisation of an acid with an alkali. Enthalpy of neutralisation is exothermic. Worked Example (Note: the method is not always identical) 50cm3 of 1 moll-1 hydrochloric acid, HCl, is mixed with 50cm3 of 1 moll-1 potassium hydroxide, KOH, both at 20oC. The temperature of the resulti ...
... formed in the neutralisation of an acid with an alkali. Enthalpy of neutralisation is exothermic. Worked Example (Note: the method is not always identical) 50cm3 of 1 moll-1 hydrochloric acid, HCl, is mixed with 50cm3 of 1 moll-1 potassium hydroxide, KOH, both at 20oC. The temperature of the resulti ...
25.4 ATP yield
... Molecules of acetyf CoA are the sam-e,regardlessof their source. Like acetyl CoA molecules produced from glucose,the acetyl CoA molecules formed in the fatty acid spiral can be oxidized in the citric acid cycle. Since we can find the yield of NADH, FADH2, and ATP from the beta-oxidation reactions of ...
... Molecules of acetyf CoA are the sam-e,regardlessof their source. Like acetyl CoA molecules produced from glucose,the acetyl CoA molecules formed in the fatty acid spiral can be oxidized in the citric acid cycle. Since we can find the yield of NADH, FADH2, and ATP from the beta-oxidation reactions of ...
Leadership Briefing Outline
... A typical PCR generates as many as 109 copies of target sequence Aerosols from pipettes will contain as many as 106 amplification products Buildup of aerosolized amplification products will contaminate laboratory reagents, equipment, and ventilation systems ...
... A typical PCR generates as many as 109 copies of target sequence Aerosols from pipettes will contain as many as 106 amplification products Buildup of aerosolized amplification products will contaminate laboratory reagents, equipment, and ventilation systems ...
c - SchoolRack
... Qualities of Water that are caused by its polarity and the biological significance of those qualities • Dissociation of water molecules leads to acidic and basic conditions that affect living organisms ...
... Qualities of Water that are caused by its polarity and the biological significance of those qualities • Dissociation of water molecules leads to acidic and basic conditions that affect living organisms ...
SOLLIQSOL questions
... Give a scientific explanation for the following observations. Use equations or diagrams if they are relevant. (a) It takes longer to cook an egg until it is hard-boiled in Denver (altitude 1 mile above sea level) than it does in New York City (near sea level). (b) Burn coal containing a significant ...
... Give a scientific explanation for the following observations. Use equations or diagrams if they are relevant. (a) It takes longer to cook an egg until it is hard-boiled in Denver (altitude 1 mile above sea level) than it does in New York City (near sea level). (b) Burn coal containing a significant ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... - are forces of attraction between atoms. - the bonding attraction comes from attractions between protons and electrons. i.) Ionic bonds - result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other - generally formed when metal atoms bonde ...
... - are forces of attraction between atoms. - the bonding attraction comes from attractions between protons and electrons. i.) Ionic bonds - result when electrons have been transferred between atoms, resulting in oppositely charged ions that attract each other - generally formed when metal atoms bonde ...
Tech Tip 0013 - Hydrates and Salts
... Confusion is not uncommon when reporting the final values calculated with using such standards. The analyst must first determine if the end result will be reported as the hydrate or salt, or as the compound without the hydrate or salt. This determines how the initial calibration curve is generated. ...
... Confusion is not uncommon when reporting the final values calculated with using such standards. The analyst must first determine if the end result will be reported as the hydrate or salt, or as the compound without the hydrate or salt. This determines how the initial calibration curve is generated. ...
Calculations with Chemical Formulas and Equations
... Analyze: We are told that isopropyl alcohol contains C, H, and O atoms and given the quantities of CO 2 and H2O produced when a given quantity of the alcohol is combusted. We must use this information to determine the empirical formula for isopropyl alcohol, a task that requires us to calculate the ...
... Analyze: We are told that isopropyl alcohol contains C, H, and O atoms and given the quantities of CO 2 and H2O produced when a given quantity of the alcohol is combusted. We must use this information to determine the empirical formula for isopropyl alcohol, a task that requires us to calculate the ...
5H2O → CuSO4 + 5H2O(g)
... state has an oxidation number of 0. 2) An atom in a monatomic ion (Na+, Cl-) has an oxidation number identical to its charge. 3a) Hydrogen has an oxidation number of +1, unless it is combined with a metal, in which case it has an oxidation number of –1. 3b) Oxygen usually has an oxidation number of ...
... state has an oxidation number of 0. 2) An atom in a monatomic ion (Na+, Cl-) has an oxidation number identical to its charge. 3a) Hydrogen has an oxidation number of +1, unless it is combined with a metal, in which case it has an oxidation number of –1. 3b) Oxygen usually has an oxidation number of ...
Topic 3
... To determine the molecular formula, we must know the molecular weight (molar mass) of the compound. ...
... To determine the molecular formula, we must know the molecular weight (molar mass) of the compound. ...
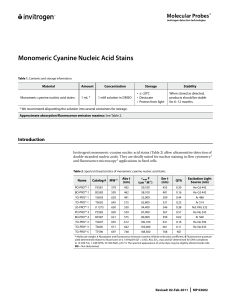
Monomeric Cyanine Nucleic Acid Stains
... Allow all solutions to warm to room temperature and mix thoroughly before use. ...
... Allow all solutions to warm to room temperature and mix thoroughly before use. ...
Free radicals
... blood and attacts a number of biological targets, acts as vasodilator, may have a role in intracellular signaling and ...
... blood and attacts a number of biological targets, acts as vasodilator, may have a role in intracellular signaling and ...
Introduction
... 1) An atom (or molecule) in its elemental state has an oxidation number of 0. 2) An atom in a monatomic ion (Na+, Cl-) has an oxidation number identical to its charge. 3a) Hydrogen has an oxidation number of +1, unless it is combined with a metal, in which case it has an oxidation number of –1. ...
... 1) An atom (or molecule) in its elemental state has an oxidation number of 0. 2) An atom in a monatomic ion (Na+, Cl-) has an oxidation number identical to its charge. 3a) Hydrogen has an oxidation number of +1, unless it is combined with a metal, in which case it has an oxidation number of –1. ...
Name: ______ Date: Period: ATP, Photosynthesis and Cellular
... Date: _________________ Period: ______________ ATP, Photosynthesis and Cellular Respiration Web quest Objective: In this web quest investigation, you will use the internet to research topics related to ATP, Photosynthesis and Cellular Respiration. Use the web links provided to answer the following q ...
... Date: _________________ Period: ______________ ATP, Photosynthesis and Cellular Respiration Web quest Objective: In this web quest investigation, you will use the internet to research topics related to ATP, Photosynthesis and Cellular Respiration. Use the web links provided to answer the following q ...
Chapter 7A- Cellular Respiration: Glycolysis - TJ
... The below figure introduces the 3 stages of cellular respiration. Label the diagram. Include electron transport chain, pyruvate, mitochondrion, citric acid cycle, glycolysis, cytoplasm, glucose, 2 NADH, 6 NADH, 2 FADH2, 2 ATP, 34 ATP, 38 ATP. ...
... The below figure introduces the 3 stages of cellular respiration. Label the diagram. Include electron transport chain, pyruvate, mitochondrion, citric acid cycle, glycolysis, cytoplasm, glucose, 2 NADH, 6 NADH, 2 FADH2, 2 ATP, 34 ATP, 38 ATP. ...
Fast and simple purification of GST fusion proteins using prepacked
... glutathione ligand is coupled via a 10-carbon linker to highly cross-linked 4% agarose. The coupling is optimized to give a high binding capacity for GST fusion proteins and other glutathione binding proteins. The total binding capacity is approximately 10 mg recombinant GST/ml gel. The dynamic bind ...
... glutathione ligand is coupled via a 10-carbon linker to highly cross-linked 4% agarose. The coupling is optimized to give a high binding capacity for GST fusion proteins and other glutathione binding proteins. The total binding capacity is approximately 10 mg recombinant GST/ml gel. The dynamic bind ...
Ch 12 Solutions
... in water because they have relatively small lattice energies. - As more solute is dissolved, fewer water molecules are available for bonding to the ions, so the overall hydration energy that is available decreases. - Hydration decreases as more solute is dissolved until it is the same as the lattice ...
... in water because they have relatively small lattice energies. - As more solute is dissolved, fewer water molecules are available for bonding to the ions, so the overall hydration energy that is available decreases. - Hydration decreases as more solute is dissolved until it is the same as the lattice ...
Gas Laws Powerpoint
... There are several laws we use to quantify the behavior of gases The laws describe some combination of changes in pressure (P), volume (V), moles/amount of gas (n),or temperature (T) Standard Temperature and Pressure (STP) ◦ 0ºC and 1 atm ◦ 1 mole of gas occupies 22.4 L ...
... There are several laws we use to quantify the behavior of gases The laws describe some combination of changes in pressure (P), volume (V), moles/amount of gas (n),or temperature (T) Standard Temperature and Pressure (STP) ◦ 0ºC and 1 atm ◦ 1 mole of gas occupies 22.4 L ...
AP Chem – Unit 1 Part 2 AP Chemistry 2016-‐2017 Unit 1
... After completion of unit 1 I will be able to… • Identify an element or determine its purity using mass percent calculations. • Use mole relationships to convert between moles, mass and particles. • ...
... After completion of unit 1 I will be able to… • Identify an element or determine its purity using mass percent calculations. • Use mole relationships to convert between moles, mass and particles. • ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.