* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Free radicals
Biosynthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Mitochondrion wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Molecular ecology wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
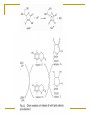
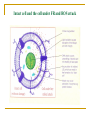
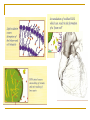
Free radical theory Oxidative stress and antioxidants in the human body The nature of free radicals Reactive oxygen, nitrogen and chlorine species The oxidative stress Important cellular sources of oxidative stress Exogenous sources of free radicals Pathological processes involving oxygen free radical reactions The defense system against free radicals and reactive oxygen species: - antioxidant enzymes, - endogenous antioxidants, - dietary antioxidants. The nature of free radicals Free radicals are highly reactive and thereby destructive molecules. A free radical is any chemical species, capable of independent (although extremely short) existence with one or more unpaired electrons. Free radicals seek electrons in order to pair their unpaired electrons. Because most of the molecules in living organism do not have unpaired electrons, free radicals steal electrons from normal molecules. This process is called oxidation. Free radicals cause a chain reaction of oxidation. The rate of radical chain reactions is incredibly high – from one billionth of a second to less than ten seconds. Radicals attack all molecules: lipids, sugars, proteins, DNA, and newly damaged molecules start a chain reaction. Many of these molecular species are oxygen (and sometimes nitrogen and chloride) centered. The molecular oxygen we breathe is a free radical. Oxidation causes physiologic damage. Free radicals are part of life, but when produced in large quantities, they can be dangerous and extremely damaging. Free radical oxidative damage has been implicated in majority of chronic diseases. The oxidative stress in living organism is defined as: the physiological state of free radicals (FR) and reactive oxygen species formation (ROS) (Helmut Sies, 1985) increased production of free radicals and reactive oxygene species causes an imbalance in the concentrations of prooxidants and antioxidants damage to intact cells and organs caused by FR and ROS Sources of FR and ROS Free radicals are formed naturally in the body – for example, as byproducts of normal metabolism, by the breakdown of bacteria by white blood cells, in enzymatic reactions. Endogenous sources: reduction of O2 (respiration) peroxidation of lipids enzymatic reactions oxidation of low molecular weight components of cells oxidation of proteins and nucleic acids in mitochondria, peroxisomes, inflammatory cells Sources of FR and ROS (cont.) They are also form outside the body and enter the body through the skin, respiration and other ways. Exogenous sources: environmental pollution (heavy metals, nitric oxides, smoking, ozone,…) radiation (electromagnetic field, UV light, ionization) sonification xenobiotic oxidation Pathological processes involving ros and fr formation The effects of free radical damage – many free radicals and reactive oxygen species have been implicated in disease development: arthritis, kidney disease, cataracts, cardiovascular disease, atherosclerosis, cancer and other malignancies, aging, degenerative neurological diseases, ischemia/reperfusion injury ….. Free radicals and reactive oxygen species overview Free radicals: Singlet O2 - extremely reactive Triplet O2 Alkoxyl radical RCO· Alkoxyl peroxide radical RCOO· Reactive oxygen species (ROS): Hydrogen peroxide H2O2 Hydroperoxyl radical HO2· Superoxide anion O2· Peroxide O22 Hydroxyl radical HO· Ozone O3 Reactive nitrogen and chlorine species overview Reactive nitrogen species (RNS): Nitric oxide NO, NO2 Peroxynitrite ONOO- Reactive chlorine species (RClS): Hypochlorous acid HOCl, hypochlorite anion OCl- Product of oxygen reduction Oxygen molecule reduction and ROS synthesis Molecular oxygen is reduced to water in four single-electron steps. Reactive oxygen species are products of individual one-electron reactions. Four electron reduction of oxygen molecule Reactice oxygen species (cont.) superoxide anion O2· - - less reactive than HO·, travels in the blood and attacts a number of biological targets, acts as vasodilator, may have a role in intracellular signaling and growth regulation, hydrogen peroxide H2O2 - crosses cellular membranes easily and may cause expression of virus genes, has only a few cellular targets but can result in the production of hydroxyl radicals, hydroxyl radical HO· - highly reactive which can attack all biological molecules, Production in vivo of hydroxyl radical: Haber-Weiss reaction: Fenton reaction: H2O2 + O2· - → HO· + OH- + O2 Fe2+ + H2O2 → HO· + OH- + Fe3+ O2· - + Fe 3+ → O2 + Fe 2+ Summerizing the Fenton reaction the H-W reaction is obtained: Fe 2+ / Fe 3+ H2O2 + O2· - → HO· + OH- + O2 Wolne rodniki … Overview of mitochondrial ROS production ROS production by mitochondria can lead to oxidative damage to mitochondrial proteins, membranes and DNA, impairing the ability of mitochondria to synthesize ATP and to carry out their wide range of metabolic functions, including the tricarboxylic acid cycle, fatty acid oxidation, the urea cycle, amino acid metabolism, haem synthesis and FeS centre assembly that are central to the normal operation of most cells. Mitochondrial oxidative damage can also increase the tendency of mitochondria to release intermembrane space proteins such as cytochrome c (cyt c) to the cytosol by mitochondrial outer membrane permeabilization (MOMP) and thereby activate the cell's apoptotic machinery. In addition, mitochondrial ROS production leads to induction of the mitochondrial permeability transition pore (PTP), which renders the inner membrane permeable to small molecules in situations such as ischaemia/reperfusion injury. Consequently, it is unsurprising that mitochondrial oxidative damage contributes to a wide range of pathologies. In addition, mitochondrial ROS may act as a modulatable redox signal, reversibly affecting the activity of a range of functions in the mitochondria, cytosol and nucleus. Biochem J. 2009 January 1; 417(Pt 1): 1–13. Oxidation of unsaturated fatty acids in lipids Product of protein oxidation: Product of DNA oxidation: Reactive nitrogen species Nitric oxides and peroxynitrite: NO, NO2, ONOO- NO is a free radical produced in our body in: the vascular endothelial cells which form the lining of blood vessels, the phagocytes which are part of the immune system, some brain cells NO acts on smooth muscle cells in vessel walls causing relaxation NO can react with O2·- and produce peroxinitrite anion ONOO-, strong oxidant, not stable at physiological pH, but its half-life time is 1 second, and it means that it can diffuse in cells The defense system against free radicals and reactive oxygen species The body is able to prevent free radical and reactive oxygen species damage by producing antioxidant molecules and enzymes. They neutralize free radicals (oxidants) by donating an electron to them or robbing one from them. Antioxidants Antioxidants are a group of compounds which, when present at low concentrations, in relation to oxidizable substrates, inhibit or delay oxidative processes, while often being oxidized themselves. The role of antioxidants is to interact with free radicals and “quench” them, or render them harmless. Antioxidants are involved in the prevention of cellular damage Preventive antioxidants – prevent the formation of new FR and ROS: Scavenging antioxidants – remove ROS once formed, thus prevent radical chain reactions: ceruloplasmin albumin transferrin, ferritin, myoglobin, haptoglobin metallothioneine enzymes: SOD, GPx, GR, CAT, paraoxonase (PON), metalloenzymes small molecules (lipophilic, hydrophilic): GSH, vitamin C, E, A (β-carotene), bilirubin, uric acid, flavonoids Repair enzymes – repair or remove ROS-damaged biomolecules: DNA repair enzymes, methionine sulphoxide reductase lipase proteases transferases Naturally occurring antioxidants, of high or low molecular weight, can differ in their composition, their physiological and chemical properties and in theirmechanism and site of action. They are divided into following categories: enzymes – attenuate the generation of reactive oxygen species by removing potential oxidants or by transforming ROS/RNS into relatively stable compounds. Superoxide dismutase - Cu,Zn-SOD, Mn-SOD, Fe-SOD SOD catalyzes the transformation of the superoxide radical into hydrogen peroxide, which can be further transformed by the enzyme catalase into water and molecular oxygen: O2· - + O2· - + 2 H+ → H2O2 + O2 2 H2O2 → H2O + O2 Naturally occurring antioxidants (cont.) Glutatione peroxidase (GPx) reduces lipid peroxides (ROOH), formed by the oxidation of polyunsaturated fatty acids (PUFA), to a stable nontoxic molecule – hydroxyl Fatty acid (ROH). ROOH + 2 GSH → ROH + H2O + GSSG Together with phospholipases, GPx can also convert phospholipids hydroperoxides (PL-OOH) into phospholipids hydroxide (PL-OH) Glutathione reductase (GR): NADPH + H+ + GSSG → NADP+ + 2 GSH Naturally occurring antioxidants (cont.) high molecular weight proteins: albumin, ceruloplasmin, transferrin, haptoglobin All are present in plasma, bind free metal ions active in the redox reactions and limit the production of metal-catalyzed free radicals. Albumin and ceruloplasmin can bind copper ions, and transferrin binds iron ions. Haptoglobin binds heme-containing proteins and can clear them from the circulation. Naturally occurring antioxidants (cont.) low molecular weight antioxidants subdivided into: lipid-soluble antioxidants (tocopherols, carotenoids, quinines, bilirubin, some polyphenols) water-soluble antioxidants (ascorbic acid, uric acid, some polyphenols) They delay or inhibit cellular damage mainly through their free radical scavenging property. Intact cell and the cell under FR and ROS attack Antioxidants as important markers of disease FR and ROS circulate freely in the body with access to all organs and tissues, which can have seriuos repercussions throughout the body. The body utilises antioxidant reserves to cope with FR and ROS and monitoring antioxidant levels may be conducive to the early detection of disease. Monitoring both individual antioxidant components and the overall status of the antioxidant system on a range of automated instruments include: Total antioxidant status (TAS) SOD activity GPx and GR activity Iron level/ total iron binding capacity (TIBC) Concentration of: transferrrin ferritin albumin bilirubin uric acid Monitoring of TAS level, SOD and GPX activity, level of selenium in some diseases Biomarkers of the oxidative stress Lipids’ damage markers: Proteins’ damage markers: lipid peroxides 4-hydroxyalkenals malonyldialdehyde (MDA) oxysterols isoprostanes, isoleucotriens carbonyl groups (>C=O, CO) adducts of CO with carbohydrates, derivatives of Tyr, Trp, Phe, His, Met, Lys, Leu, Ile, Val oxidation, nitration, chlorination oxidation of –SH groups: sulphoxides = S(O)2 Markers of DNA (purine and pyrimidine bases) damage: deamination products, adducts with CO nitration and oxidation products