Synthetic Polymers - McQuarrie General Chemistry
... tendency of individual polymer chains is to coil into the shape of a ball, as shown in Figure S.5a. If we were to grab the polymer at two points and stretch it, it would rebound into the coiled configuration upon release. Thus, individual polymer chains show a property of elasticity. In a sample of ...
... tendency of individual polymer chains is to coil into the shape of a ball, as shown in Figure S.5a. If we were to grab the polymer at two points and stretch it, it would rebound into the coiled configuration upon release. Thus, individual polymer chains show a property of elasticity. In a sample of ...
Lifeline Week 6 Follow-Along Sheet Cellular Respiration
... __________ and ____________ are coenzymes that are _______________ (have gained an electron and a proton). NAD+ and FADH are the _________________ form of the coenzymes (have lost an electron and a proton). These coenzymes are electron carriers that are required for cellular respiration to take ...
... __________ and ____________ are coenzymes that are _______________ (have gained an electron and a proton). NAD+ and FADH are the _________________ form of the coenzymes (have lost an electron and a proton). These coenzymes are electron carriers that are required for cellular respiration to take ...
F.Y. B.Sc. - Vocational Biotechnology
... Partition principle, Thin layer chromatography , Paper chromatography , Ion exchange chromatography , Affinity chromatography ,Gel filtration chromatography Spectrophotometry 1) uv and visible spectrophotometry - With Basics 2) Nephelometry 3) Turbidometry Microscopy 1) Introduction to microscopy 2) ...
... Partition principle, Thin layer chromatography , Paper chromatography , Ion exchange chromatography , Affinity chromatography ,Gel filtration chromatography Spectrophotometry 1) uv and visible spectrophotometry - With Basics 2) Nephelometry 3) Turbidometry Microscopy 1) Introduction to microscopy 2) ...
IDENTIFICATION OF LEAD COMPOUNDS WITH COBRA VENOM NEUTRALISING ACTIVITY IN
... effects, cost effective and need not require sophisticated storage system when compared to the antivenom therapy. There is also a growing realization that diseases have multifactorial causation, a combination of drugs acting at a number of targets simultaneously is likely to be more effective than d ...
... effects, cost effective and need not require sophisticated storage system when compared to the antivenom therapy. There is also a growing realization that diseases have multifactorial causation, a combination of drugs acting at a number of targets simultaneously is likely to be more effective than d ...
HIGH SCHOOL CHEMISTRY REVIEW LECTURE 2: REACTION
... REALLY SALTY? Clearly we need some QUANTITATIVE measure of solute concentration. There are many ways to do this. I give you the two most common. Percent by mass This measure of concentration is used because it follows simply from doing a measurement on a balance. ...
... REALLY SALTY? Clearly we need some QUANTITATIVE measure of solute concentration. There are many ways to do this. I give you the two most common. Percent by mass This measure of concentration is used because it follows simply from doing a measurement on a balance. ...
Chapter 5 Polypeptides Geometry of Peptide Bond
... (for sodium dodecylsulfate). This strong detergent is able to completely disrupt the hydrophobic bonding of the protein and allow the protein to unfold to an extended structure. The SDS molecules bind to the extended structure at a ratio of about 1 SDS molecule for each amino acid residue, or about ...
... (for sodium dodecylsulfate). This strong detergent is able to completely disrupt the hydrophobic bonding of the protein and allow the protein to unfold to an extended structure. The SDS molecules bind to the extended structure at a ratio of about 1 SDS molecule for each amino acid residue, or about ...
Topic 1: Quantitative chemistry
... 3. Law of Combining Volumes of Gases: Gay-Lussac (1803) concluded that when gases react they do so in whole number ratios in terms of volume e.g. 3 volumes of hydrogen react with 1 volume of nitrogen to form 2 volumes of ammonia. 4. Avogadro’s Theory (1811): Avogadro stated that equal volumes of gas ...
... 3. Law of Combining Volumes of Gases: Gay-Lussac (1803) concluded that when gases react they do so in whole number ratios in terms of volume e.g. 3 volumes of hydrogen react with 1 volume of nitrogen to form 2 volumes of ammonia. 4. Avogadro’s Theory (1811): Avogadro stated that equal volumes of gas ...
Document
... – Hydroxyl – in alcohols, sugar – Carbonyl – in sugars, amino acids, nucleotide bases – Carboxyl – in amino acids, fatty acids; acts as an acid and releases H+ – Amino – in amino acids; acts as a weak base – Sulfhydryl – in amino acid cysteine; helps stabilize protein ...
... – Hydroxyl – in alcohols, sugar – Carbonyl – in sugars, amino acids, nucleotide bases – Carboxyl – in amino acids, fatty acids; acts as an acid and releases H+ – Amino – in amino acids; acts as a weak base – Sulfhydryl – in amino acid cysteine; helps stabilize protein ...
Chapter 5 Polypeptides Geometry of Peptide Bond
... completely disrupt the hydrophobic bonding of the protein and allow the protein to unfold to an extended structure. The SDS molecules bind to the extended structure at a ratio of about 1 SDS molecule for each amino acid residue, or about 1.4 g SDS per g protein. Rod-like structures are formed, where ...
... completely disrupt the hydrophobic bonding of the protein and allow the protein to unfold to an extended structure. The SDS molecules bind to the extended structure at a ratio of about 1 SDS molecule for each amino acid residue, or about 1.4 g SDS per g protein. Rod-like structures are formed, where ...
Final Review
... a. the amount of energy required to rearrange chemical bonds. b. the amount of energy required to initiate a reaction. c. the number of chemical bonds which are changed during a reaction. d. the state of equilibrium in a system. e. the amount of disorder in a system. 90. All of the statements are tr ...
... a. the amount of energy required to rearrange chemical bonds. b. the amount of energy required to initiate a reaction. c. the number of chemical bonds which are changed during a reaction. d. the state of equilibrium in a system. e. the amount of disorder in a system. 90. All of the statements are tr ...
Final Exam Practice Questions for General Chemistry NOTICE TO
... familiar with the TYPES of questions that are generally presented on the final exam. This is not a complete list of the ideas, concepts, principles, or questions covered in the general chemistry class. Be aware that there are many other questions and ideas that may not be covered in this list. This ...
... familiar with the TYPES of questions that are generally presented on the final exam. This is not a complete list of the ideas, concepts, principles, or questions covered in the general chemistry class. Be aware that there are many other questions and ideas that may not be covered in this list. This ...
INERT GASES -
... I t was then realized that these gases formed an entirely new group in the periodic table - elements which were characterized by complete chemical inactivity. Apart from radon, which is radioactive, and helium, which exhibits unusual properties at low temperatures, the inert gases had little obvious ...
... I t was then realized that these gases formed an entirely new group in the periodic table - elements which were characterized by complete chemical inactivity. Apart from radon, which is radioactive, and helium, which exhibits unusual properties at low temperatures, the inert gases had little obvious ...
AP Chemistry Summer Assignment 2016
... before we start this actual unit during the school year. If you have a demanding schedule this fall or struggled with this unit last year, I recommend that you work your way through the preassignment for Chapter 5 this summer. Most of Chapter 5 is review, we will not spend much class time on Chapter ...
... before we start this actual unit during the school year. If you have a demanding schedule this fall or struggled with this unit last year, I recommend that you work your way through the preassignment for Chapter 5 this summer. Most of Chapter 5 is review, we will not spend much class time on Chapter ...
Empirical and Molecular Formulas and Percentage Composition
... The molar mass of a substance is determined the same way, but you are finding the mass of _________________________ instead of the mass of just one _______________ (for a covalent compound) or one _____________________ (for an ionic compound). molar mass of NaCl = ________ g ...
... The molar mass of a substance is determined the same way, but you are finding the mass of _________________________ instead of the mass of just one _______________ (for a covalent compound) or one _____________________ (for an ionic compound). molar mass of NaCl = ________ g ...
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) ISSN: 2278-3008.
... namely α, β and ε [7] AccD5 plays major role in cell envelope lipid biosynthesis and its disruption lead to pathogen death. In molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex. Knowledge of ...
... namely α, β and ε [7] AccD5 plays major role in cell envelope lipid biosynthesis and its disruption lead to pathogen death. In molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex. Knowledge of ...
W2(SO4)3 + Mg3(PO4)2 --------> WPO4 + MgSO4
... For each example, you must solve for each stoichiometry reaction. Remember, stoichiometry is the study of the quantities of reactants and products in a chemical process. The key to stoichiometry is the mole ratio, the balanced coefficients in front of each product and reactant in a given reaction. F ...
... For each example, you must solve for each stoichiometry reaction. Remember, stoichiometry is the study of the quantities of reactants and products in a chemical process. The key to stoichiometry is the mole ratio, the balanced coefficients in front of each product and reactant in a given reaction. F ...
Thin-Layer Chromatography of Amino Acids
... chromatography, gas chromatography, liquid chromatography, and thinlayer chromatography. Thin Layer Chromatography (TLC) is used to separate solids from a liquid. The most common use is to separate amino acids from a liquid and each other. A spot of the sample is placed on a sheet of glass treated w ...
... chromatography, gas chromatography, liquid chromatography, and thinlayer chromatography. Thin Layer Chromatography (TLC) is used to separate solids from a liquid. The most common use is to separate amino acids from a liquid and each other. A spot of the sample is placed on a sheet of glass treated w ...
Problem 1: A brief history of life in the universe
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
6.1 Moles and Molar Masses
... Empirical formulas can be calculated from lab data, allowing us to identify unknown compounds: STEP 1: Assume mass percentages represent masses, in g: STEP 2: Divide each element's mass by their respective molar masses, turning them into moles. STEP 3: Divide all moles by the lowest number of moles ...
... Empirical formulas can be calculated from lab data, allowing us to identify unknown compounds: STEP 1: Assume mass percentages represent masses, in g: STEP 2: Divide each element's mass by their respective molar masses, turning them into moles. STEP 3: Divide all moles by the lowest number of moles ...
Problem 1: “A brief history” of life in the universe
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
Problem 1: “A brief history” of life in the universe
... Problem 3: Spectroscopy of interstellar molecules Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different s ...
... Problem 3: Spectroscopy of interstellar molecules Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different s ...
... The doped polymer is thus a salt. However, it is not the counter ions, I3– or Na+, but the charges on the polymer that are the mobile charge carriers (see Mechanism of polymer conductivity, below). By applying an electric field perpendicular to the film, the counter ions can be made to diffuse from ...
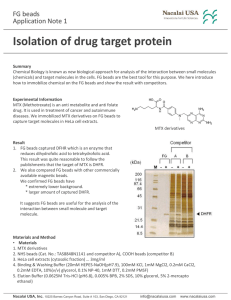
Isolation of drug target protein
... MTX (Methotrexate) is an anti metabolite and anti folate drug. It is used in treatment of cancer and autoimmune diseases. We immobilized MTX derivatives on FG beads to capture target molecules in HeLa cell extracts. MTX derivatives Result 1. FG beads captured DFHR which is an enzyme that reduces dih ...
... MTX (Methotrexate) is an anti metabolite and anti folate drug. It is used in treatment of cancer and autoimmune diseases. We immobilized MTX derivatives on FG beads to capture target molecules in HeLa cell extracts. MTX derivatives Result 1. FG beads captured DFHR which is an enzyme that reduces dih ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... Regardless, the dissolved Oxygen gas in water allows for life forms to live in the water. They mainly use gills or diffusion to get the Oxygen gas out of the water. 4. London Dispersion Forces (Non-polar molecules) a. These were discovered by Fritz London in 1930. b. They are also known as Van der ...
... Regardless, the dissolved Oxygen gas in water allows for life forms to live in the water. They mainly use gills or diffusion to get the Oxygen gas out of the water. 4. London Dispersion Forces (Non-polar molecules) a. These were discovered by Fritz London in 1930. b. They are also known as Van der ...
Final Exam Review
... a. the amount of energy required to rearrange chemical bonds. b. the amount of energy required to initiate a reaction. c. the number of chemical bonds which are changed during a reaction. d. the state of equilibrium in a system. e. the amount of disorder in a system. 90. All of the statements are tr ...
... a. the amount of energy required to rearrange chemical bonds. b. the amount of energy required to initiate a reaction. c. the number of chemical bonds which are changed during a reaction. d. the state of equilibrium in a system. e. the amount of disorder in a system. 90. All of the statements are tr ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.