study material(2014-15) class xii-chemistry
... Tips and Techniques for teaching/learning each chapter. Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
... Tips and Techniques for teaching/learning each chapter. Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
chemistry-resource
... Tips and Techniques for teaching/learning each chapter. Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
... Tips and Techniques for teaching/learning each chapter. Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among the ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... number of molecules as well as the number of moles of each substance ...
... number of molecules as well as the number of moles of each substance ...
Insurance Premiums Order (1993-94)
... (1) In this Order:"basic tariff premium", in relation to a policy, means the basic tariff premium for the policy calculated in accordance with Schedule 3."category A employer", in relation to a policy, means an employer whose basic tariff premium for the policy at the time at which the insurer first ...
... (1) In this Order:"basic tariff premium", in relation to a policy, means the basic tariff premium for the policy calculated in accordance with Schedule 3."category A employer", in relation to a policy, means an employer whose basic tariff premium for the policy at the time at which the insurer first ...
Ch 18 Power Point
... The Equilibrium Expression, continued The H2, I2, HI Equilibrium System • The rate of the reaction between H2 and I2 vapor in a sealed flask at an elevated temperature can be followed by observing the rate at which the violet color of the iodine vapor diminishes. • The color fades to a constant inte ...
... The Equilibrium Expression, continued The H2, I2, HI Equilibrium System • The rate of the reaction between H2 and I2 vapor in a sealed flask at an elevated temperature can be followed by observing the rate at which the violet color of the iodine vapor diminishes. • The color fades to a constant inte ...
Chemistry Final Exam Review
... ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion reaction will always yield water as a product. ____ 29. A single displacement reaction has two comp ...
... ____ 25. Subscripts are used to balance chemical reactions. ____ 26. A synthesis reaction contains two products. ____ 27. A decomposition reaction contains at least two products. ____ 28. A combustion reaction will always yield water as a product. ____ 29. A single displacement reaction has two comp ...
Cookies and Chemistry…Huh!?!?
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
Chapter+12
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
Fundamentals
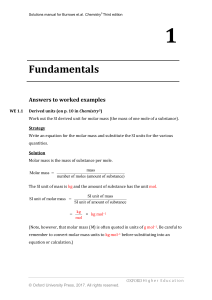
... Write a full, balanced equation for the reaction, showing the ions present in the reactants and products. Identify any non-ionic substances and include state symbols in the equation. Cross out the spectator ions that appear on both sides of the equation and so do not take part in the reaction. Solut ...
... Write a full, balanced equation for the reaction, showing the ions present in the reactants and products. Identify any non-ionic substances and include state symbols in the equation. Cross out the spectator ions that appear on both sides of the equation and so do not take part in the reaction. Solut ...
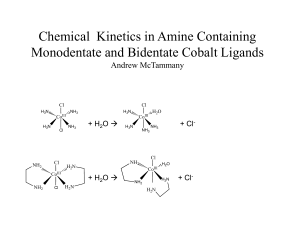
Chemical Kinetics in Monodentate and Bidentate Cobalt Compounds
... repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evaluated. ...
... repeated. The trans-Co(NH3)4Cl2 should be synthesized using a lower temperature and lower concentration of acid. A mixture of HCl and H2SO4 could be used instead. From there the effect of different ligands can be evaluated. ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... Chemical equations help us make predictions about chemical reactions without having to run the reactions in the laboratory. The reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal ...
... Chemical equations help us make predictions about chemical reactions without having to run the reactions in the laboratory. The reaction-stoichiometry calculations described in this chapter are theoretical. They tell us the amounts of reactants and products for a given chemical reaction under ideal ...
Stoichiometry - Social Circle City Schools
... of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit chemists use when counting numbers of atoms or molecules in a sample. The number of parti ...
... of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit chemists use when counting numbers of atoms or molecules in a sample. The number of parti ...
PFC ,RR-86-1 The Kinetics Of Liquid Lithium Reaction With Oxygen
... and LiPb compounds is shown in Table 1.2. Due to the potentially large amount of hot flowing lithium in fusion reactors, one must be aware of the hazards of lithium leaks and spills. Presently, significant effort is dedicated to characterizing the reaction kinetics of both lithium and lithium-lead c ...
... and LiPb compounds is shown in Table 1.2. Due to the potentially large amount of hot flowing lithium in fusion reactors, one must be aware of the hazards of lithium leaks and spills. Presently, significant effort is dedicated to characterizing the reaction kinetics of both lithium and lithium-lead c ...
Objectives - hartman
... product that can be produced from a given amount of reactant. • The actual yield of a product is the measured amount of that product obtained from a reaction. • The percentage yield is the ratio of the actual yield to the theoretical yield, multiplied by 100. ...
... product that can be produced from a given amount of reactant. • The actual yield of a product is the measured amount of that product obtained from a reaction. • The percentage yield is the ratio of the actual yield to the theoretical yield, multiplied by 100. ...
CHAPTER 12 | The Chemistry of Solids
... Drawings b and d are analogous to crystalline solids because they show a definite pattern while a and c are amorphous. Think about It In drawings b and d there are two kinds of atoms; if the drawings represent metals, these two substances would be alloys. 12.3. Collect and Organize Using Figure P12. ...
... Drawings b and d are analogous to crystalline solids because they show a definite pattern while a and c are amorphous. Think about It In drawings b and d there are two kinds of atoms; if the drawings represent metals, these two substances would be alloys. 12.3. Collect and Organize Using Figure P12. ...











![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)