
Problem Authors - PianetaChimica
... contaminations. The reaction of sulfuric acid with colemanite takes place in two steps: In the first step colemanite is dissolved in sulfuric acid forming the calcium(II) ion and boric acid. In the second step, calcium sulfate, formed from Ca2+ and SO42− ions, precipitates as gypsum crystals. In an ...
... contaminations. The reaction of sulfuric acid with colemanite takes place in two steps: In the first step colemanite is dissolved in sulfuric acid forming the calcium(II) ion and boric acid. In the second step, calcium sulfate, formed from Ca2+ and SO42− ions, precipitates as gypsum crystals. In an ...
Chapter 3 2013
... What mass of carbon dioxide can be produced by the reaction of 0.540 mole of iron (III) oxide with excess carbon monoxide? key riff! It tells you one reactant is in excess and the other is not! ...
... What mass of carbon dioxide can be produced by the reaction of 0.540 mole of iron (III) oxide with excess carbon monoxide? key riff! It tells you one reactant is in excess and the other is not! ...
Stoichiometry Worksheet
... 8. The average human requires 120.0 grams of glucose (C6H12O6) per day. How many grams of CO2 (in the photosynthesis reaction) are required for this amount of glucose? The photosynthetic reaction is: 6 CO2 + 6 H2O C6H12O6 + 6 O2 This problem is slightly different from those above. ...
... 8. The average human requires 120.0 grams of glucose (C6H12O6) per day. How many grams of CO2 (in the photosynthesis reaction) are required for this amount of glucose? The photosynthetic reaction is: 6 CO2 + 6 H2O C6H12O6 + 6 O2 This problem is slightly different from those above. ...
Rutile titanium dioxide nanoparticles and ordered acicular
... In a preferred embodiment of the invention, the morphol ogy controlling agent is mandelic acid (C6H5CH(OH) COOH), and the soluble titanium compound is titanium oxy ...
... In a preferred embodiment of the invention, the morphol ogy controlling agent is mandelic acid (C6H5CH(OH) COOH), and the soluble titanium compound is titanium oxy ...
Oxidative Alihatic Carbon-Carbon Bond Cleavage Reactions
... biomass for fuel production, applications in wastewater treatment and bioremediation, and in developing new reactions for organic synthesis of fine chemicals including pharmaceuticals. Ideally these reactions would be carried out with high atom economy at low temperatures and pressures, and using ea ...
... biomass for fuel production, applications in wastewater treatment and bioremediation, and in developing new reactions for organic synthesis of fine chemicals including pharmaceuticals. Ideally these reactions would be carried out with high atom economy at low temperatures and pressures, and using ea ...
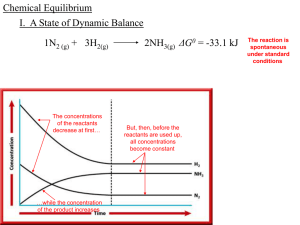
Chemical Equilibrium - local.brookings.k12.sd.us
... II. Equilibrium Expressions -_________ equilibria in which all ________ reactants and and Constants ________ products are in the same ________ physical _____ state are ____________, homogeneous but reactions with _________ reactants and ________ products in _____ more than ___ one ________ physical ...
... II. Equilibrium Expressions -_________ equilibria in which all ________ reactants and and Constants ________ products are in the same ________ physical _____ state are ____________, homogeneous but reactions with _________ reactants and ________ products in _____ more than ___ one ________ physical ...
CO 2 - TrimbleChemistry
... • A compound is represented by using the symbols for the elements of which it is composed • Subscripts are used to indicate how many atoms of a particular element exist in the compound • If there is only one atom of a particular element, the one is assumed ...
... • A compound is represented by using the symbols for the elements of which it is composed • Subscripts are used to indicate how many atoms of a particular element exist in the compound • If there is only one atom of a particular element, the one is assumed ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... with TBHP (in decane) as oxidant in the MeCN/Toluene system. High activities were observed under mild conditions with catalyst loadings as low as %1 and with high chemoselectivities for the epoxidation of cyclooctene. The rate law for the catalyzed process has been derived and the difference with re ...
... with TBHP (in decane) as oxidant in the MeCN/Toluene system. High activities were observed under mild conditions with catalyst loadings as low as %1 and with high chemoselectivities for the epoxidation of cyclooctene. The rate law for the catalyzed process has been derived and the difference with re ...
Chapter 1: Chemistry: The Study of Change
... E) No reaction occurs when the solutions are mixed. Ans: B Category: Medium Section: 4.2 7. What is the correct formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium hydroxide? A) CaO B) CaCl2 C) CaH2 D) CaCl E) CaClH Ans: B Category: Medium Section: 4.3 8. What ...
... E) No reaction occurs when the solutions are mixed. Ans: B Category: Medium Section: 4.2 7. What is the correct formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium hydroxide? A) CaO B) CaCl2 C) CaH2 D) CaCl E) CaClH Ans: B Category: Medium Section: 4.3 8. What ...
(K c ) [A] - Knockhardy
... “If the concentrations of all the substances present at equilibrium are raised to the power of the number of moles they appear in the equation, the product of the concentrations of the products divided by the product of the concentrations of the reactants is a constant, provided the temperature rema ...
... “If the concentrations of all the substances present at equilibrium are raised to the power of the number of moles they appear in the equation, the product of the concentrations of the products divided by the product of the concentrations of the reactants is a constant, provided the temperature rema ...
Vinnitsa National Pirogov Memorial Medical University Biological
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
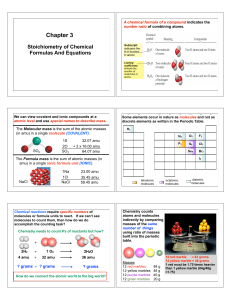
Stoichiometry
... The limiting reagent is used to determine the maximum yield of product/s aka the theoretical yield and the maximum consumption of reactants Identifying Limiting Reagents: 1. Convert all given values into moles 2. Divide each mole value by the coefficient 3. The smallest number identifies the LR ...
... The limiting reagent is used to determine the maximum yield of product/s aka the theoretical yield and the maximum consumption of reactants Identifying Limiting Reagents: 1. Convert all given values into moles 2. Divide each mole value by the coefficient 3. The smallest number identifies the LR ...
LaBrake, Fundamentals Diagnostic Questions
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
Answers - logo Pre-U Chemistry Textbook
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
... which leads to a bond angle of 120°. The nitrogen in ammonia has three bonding pairs and one lone pair of electrons. The repulsion is greatest between the lone pair and the bonding pairs. This leads to a squashing of the tetrahedral angle leading to a pyramidal shape with an HNH bond angle of 107°. ...
Chapter 09 An Overview of Chemical Reactions Notes
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
Document
... – Consider ethanol, which has the formula C2H5OH. The mass of each element present and the molar mass are obtained through the following procedure: Mass of C = 2 mol 12.011 g/mol = 24.022 g Mass of H = 6 mol 1.008 g/mol = 6.048 g Mass of O = 1 mol 15.999 g/mol = 15.999 g Mass of 1 mol of C ...
... – Consider ethanol, which has the formula C2H5OH. The mass of each element present and the molar mass are obtained through the following procedure: Mass of C = 2 mol 12.011 g/mol = 24.022 g Mass of H = 6 mol 1.008 g/mol = 6.048 g Mass of O = 1 mol 15.999 g/mol = 15.999 g Mass of 1 mol of C ...













![(K c ) [A] - Knockhardy](http://s1.studyres.com/store/data/011755527_1-914ea907d1ff7656ef398ad87316c94c-300x300.png)






