Ch. 12 Stoichiometry
... Moles: Coefficients of balanced equation indicate relative # of moles of reactant and products / Most important info from equation Mass: Obeys law of conservation of mass ...
... Moles: Coefficients of balanced equation indicate relative # of moles of reactant and products / Most important info from equation Mass: Obeys law of conservation of mass ...
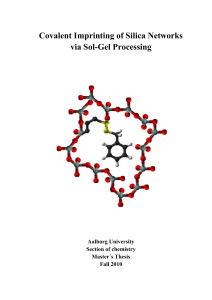
Chemistry (Revised)
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
2013 - SQA
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
Chapter 12
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
MULTIPLY CHOICE QUESTIONS ON MEDICAL CHEMISTRY
... 2.24. How will change the rate of reaction 2CO(g) + O2(g) = 2CO2(g), if the initial concentration increase in three times? А. increase in 3 times B. increase in 6 times C. does not change D. increase in 27 times E. increase in 9 times 2.25. According to the reaction СаСО3(s) → СаО(s) + СО2(g) corre ...
... 2.24. How will change the rate of reaction 2CO(g) + O2(g) = 2CO2(g), if the initial concentration increase in three times? А. increase in 3 times B. increase in 6 times C. does not change D. increase in 27 times E. increase in 9 times 2.25. According to the reaction СаСО3(s) → СаО(s) + СО2(g) corre ...
Balancing Chemical Equations Using Models
... 6. Then add any coefficients if they are needed, and count how many atoms we now have. ___HCl + ____NaOH ____NaCl + ____H2O For our example we have the same number of each atom for both products and reactants, we do not need to add any coefficients to balance the equation. Reactants Before Balancing ...
... 6. Then add any coefficients if they are needed, and count how many atoms we now have. ___HCl + ____NaOH ____NaCl + ____H2O For our example we have the same number of each atom for both products and reactants, we do not need to add any coefficients to balance the equation. Reactants Before Balancing ...
Chapter 1 - Solutions
... 84) Define limiting reactant and excess reactant. What is the significance of the limiting reactant in predicting the amount of product obtained in a reaction? Can there be a limiting reactant if only one reactant is present? The limiting reactant is the reactant that first runs out in a chemical r ...
... 84) Define limiting reactant and excess reactant. What is the significance of the limiting reactant in predicting the amount of product obtained in a reaction? Can there be a limiting reactant if only one reactant is present? The limiting reactant is the reactant that first runs out in a chemical r ...
FREE Sample Here
... A) atoms must be balanced on both sides of the reaction arrow. B) mass must be conserved. C) molecules must be balanced on both sides of the reaction arrow. D) net charge must be balanced on both sides of the reaction arrow. Answer: C Diff: 1 Topic: Section 6.2 Balancing Chemical Equations 2) Which ...
... A) atoms must be balanced on both sides of the reaction arrow. B) mass must be conserved. C) molecules must be balanced on both sides of the reaction arrow. D) net charge must be balanced on both sides of the reaction arrow. Answer: C Diff: 1 Topic: Section 6.2 Balancing Chemical Equations 2) Which ...
chemistry - Brilliant Public School Sitamarhi
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
Stoichiometry and the Mole
... Curiously, this chemical reaction question is very similar to the pound cake question. Both of them involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiome ...
... Curiously, this chemical reaction question is very similar to the pound cake question. Both of them involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiome ...
Stoichiometric Calculations
... 2 molecules of H2 plus 1 molecule of O2 yields 2 molecules of H2 O. Note...while moles can be expressed as non-whole numbers, particles must be whole numbers. One cannot have 6.1 atoms, molecules, or formula units! ...
... 2 molecules of H2 plus 1 molecule of O2 yields 2 molecules of H2 O. Note...while moles can be expressed as non-whole numbers, particles must be whole numbers. One cannot have 6.1 atoms, molecules, or formula units! ...
2 - cloudfront.net
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
Kinetic isotope effects of 12CH3D+OH and 13CH3D+OH from 278 to
... tively. The kinetic isotope effect C,D α for the reaction with OH is not described in the existing literature. The related kinetic isotope effect for the CH4 + Cl reaction was measured at room temperature with the present setup by Joelsson et al. (2014) and found to be 1.60 ± 0.04. The kinetic isoto ...
... tively. The kinetic isotope effect C,D α for the reaction with OH is not described in the existing literature. The related kinetic isotope effect for the CH4 + Cl reaction was measured at room temperature with the present setup by Joelsson et al. (2014) and found to be 1.60 ± 0.04. The kinetic isoto ...