File - Flipped Out Science with Mrs. Thomas!
... 1. Complete this test review and then study! 2. Text book pages that relate to this material: Unit 3 – p. 109 – 135 and 139 – 168 (the second half of the unit focuses on the second half of Unit 3 in the textbook) 3. Notes are always posted on the website – look in Unit 2.2 4. Remember, the “Unit Enr ...
... 1. Complete this test review and then study! 2. Text book pages that relate to this material: Unit 3 – p. 109 – 135 and 139 – 168 (the second half of the unit focuses on the second half of Unit 3 in the textbook) 3. Notes are always posted on the website – look in Unit 2.2 4. Remember, the “Unit Enr ...
CHEM MINI-COURSE SERIES M1.2___
... This equation as written is unbalanced. On the left side of the arrow (reactant side), there are 2 atoms of oxygen. On the right side of the arrow (product side), there is only one atom of oxygen. A quick-and-easy attempt to balance the two sides may be to add a subscript to the H2O formula, so it a ...
... This equation as written is unbalanced. On the left side of the arrow (reactant side), there are 2 atoms of oxygen. On the right side of the arrow (product side), there is only one atom of oxygen. A quick-and-easy attempt to balance the two sides may be to add a subscript to the H2O formula, so it a ...
Acid Rain - Controlled Assessment
... Why do chemists want to know the rate of a reaction? If you are making a product, it is important to know how long the reaction takes to complete, before the product is produced. Rate is a measure of a change that happens over a single unit time. That unit time is most often a second, a minute, or a ...
... Why do chemists want to know the rate of a reaction? If you are making a product, it is important to know how long the reaction takes to complete, before the product is produced. Rate is a measure of a change that happens over a single unit time. That unit time is most often a second, a minute, or a ...
MATTER-Ch. 3-homogeneous vs. heterogeneous, elements
... Which part of an atom has a mass approximately equal to 1/2000 of the mass of a common hydrogen atom? a. nucleus c. proton b. electron d. electron cloud ____ 26. The mass of a neutron is a. about the same as that of a proton. c. double that of a proton. b. about the same as that of an electron. d. d ...
... Which part of an atom has a mass approximately equal to 1/2000 of the mass of a common hydrogen atom? a. nucleus c. proton b. electron d. electron cloud ____ 26. The mass of a neutron is a. about the same as that of a proton. c. double that of a proton. b. about the same as that of an electron. d. d ...
9 free IB Chem labs (sent to OCC) - VicPark-IBRoundtable-2009
... measuring the temperature for a few minutes to let the temperature cool down a bit. 7. Dispose of the materials in the inorganic waste beaker and wash out the equipment carefully. 8. Repeat this experiment a total of 3 times. Data Collection and Processing: Since we are using data logging software, ...
... measuring the temperature for a few minutes to let the temperature cool down a bit. 7. Dispose of the materials in the inorganic waste beaker and wash out the equipment carefully. 8. Repeat this experiment a total of 3 times. Data Collection and Processing: Since we are using data logging software, ...
Document
... How many moles of CO2 can be produced from the combustion of 8.7 mol octane (C8H18)? (Use coefficients from balanced chemical equation to make a mole ratio) ...
... How many moles of CO2 can be produced from the combustion of 8.7 mol octane (C8H18)? (Use coefficients from balanced chemical equation to make a mole ratio) ...
MIDDLE COLLEGE HIGH SCHOOL
... 5.0-gram strip of zinc because the powdered (3) effective collisions between reacting zinc has particles (1) lower kinetic energy (4) addition of a catalyst to the reaction system (2) lower concentration 8. Given the potential energy diagram for a (3) more surface area chemical reaction: (4) more zi ...
... 5.0-gram strip of zinc because the powdered (3) effective collisions between reacting zinc has particles (1) lower kinetic energy (4) addition of a catalyst to the reaction system (2) lower concentration 8. Given the potential energy diagram for a (3) more surface area chemical reaction: (4) more zi ...
Chapter 12 Review “Stoichiometry”
... Which of the following is NOT true about “yield”? a) the value of actual yield must be known to calculate percent yield, or b) the actual yield may be different from the theoretical yield ...
... Which of the following is NOT true about “yield”? a) the value of actual yield must be known to calculate percent yield, or b) the actual yield may be different from the theoretical yield ...
Full answers
... Step 2 is rate determining step and this will determine the rate of the reaction. The subsequent step can be ignored in working out the rate. Step 2 involves the decomposition of N2O4 and depends only on its concentration: rate = k2[N2O4(g)] As this involves the concentration of a reaction intermedi ...
... Step 2 is rate determining step and this will determine the rate of the reaction. The subsequent step can be ignored in working out the rate. Step 2 involves the decomposition of N2O4 and depends only on its concentration: rate = k2[N2O4(g)] As this involves the concentration of a reaction intermedi ...
Chapter 8
... designates a substance in the gaseous phase, placed after the formula designates an aqueous solution, one that is dissolved in water, placed after the formula. 8. __________ indicates that heat is supplied to the reaction 9. __________ a formula written above or below the yield sign indicates it is ...
... designates a substance in the gaseous phase, placed after the formula designates an aqueous solution, one that is dissolved in water, placed after the formula. 8. __________ indicates that heat is supplied to the reaction 9. __________ a formula written above or below the yield sign indicates it is ...
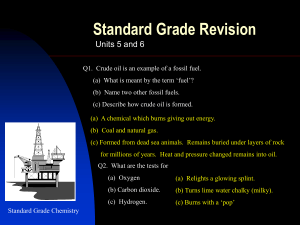
Unit 5 and 6 revsion - Deans Community High School
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
Final review free response ch 1-4
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...
... 5. If you have 4 g NaOH, and 10 g HBr, what is the limiting reagent and how much salt is produced? In lab if you produce1 g salt, what is the percent yield? ...