Part II - American Chemical Society
... Part II of this test requires that student answers be written in a response booklet of blank pages. Only this “Blue Book” is graded for a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing ma ...
... Part II of this test requires that student answers be written in a response booklet of blank pages. Only this “Blue Book” is graded for a score on Part II. Testing materials, scratch paper, and the “Blue Book” should be made available to the student only during the examination period. All testing ma ...
Master Equation Solver for Multi-Energy well Reactions
... 1) OH + CH3COCHO → H2O + CH3COCO CH3COCO → CH3CO + CO CH3COCO retains sufficient energy such that the acetyl fragment can also dissociate.4 2) OH + C2H2/O2 → (HCO)2 + OH → HCOOH + HCO OH yield dependent on fraction of O2, i.e. whether O2 reacts with chemically activated OH adduct.5 • New class of re ...
... 1) OH + CH3COCHO → H2O + CH3COCO CH3COCO → CH3CO + CO CH3COCO retains sufficient energy such that the acetyl fragment can also dissociate.4 2) OH + C2H2/O2 → (HCO)2 + OH → HCOOH + HCO OH yield dependent on fraction of O2, i.e. whether O2 reacts with chemically activated OH adduct.5 • New class of re ...
No Slide Title
... • If the initial concentration of PCl5 is 0.100 M, and Kc = 0.60, what are the equilibrium concentrations for the reaction? First, calculate y, the change in concentration. • PCl5 (g) ⇌ PCl3(g) + Cl2(g) • y = 0.087 or -0.687 Choose 0.087 to get a result in which all the concentrations are positive ...
... • If the initial concentration of PCl5 is 0.100 M, and Kc = 0.60, what are the equilibrium concentrations for the reaction? First, calculate y, the change in concentration. • PCl5 (g) ⇌ PCl3(g) + Cl2(g) • y = 0.087 or -0.687 Choose 0.087 to get a result in which all the concentrations are positive ...
Prelab Assignment: The lodination of Acetone
... The rate of the reaction equals the initial concentration of 1, in the reaction mixture divided by the elapsed time. Since the reaction is zero order in I, and since both acetone and H+ ion are present in great excess, the rate is constant throughout the reaction and the concentrations of both aceto ...
... The rate of the reaction equals the initial concentration of 1, in the reaction mixture divided by the elapsed time. Since the reaction is zero order in I, and since both acetone and H+ ion are present in great excess, the rate is constant throughout the reaction and the concentrations of both aceto ...
Balancing Equations
... side. Compare those against the number of the atoms of the same element on the right side. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. Check your answer to see if: n ...
... side. Compare those against the number of the atoms of the same element on the right side. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. Check your answer to see if: n ...
Formula Equation - Chemistry Teaching Resources
... Remember that the Roman numeral tells you the number of bonds, it does not tell you how many atoms should be in the formula. Test Yourself ...
... Remember that the Roman numeral tells you the number of bonds, it does not tell you how many atoms should be in the formula. Test Yourself ...
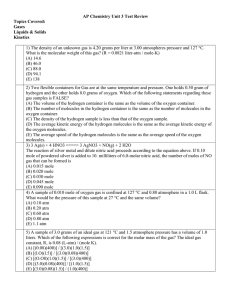
AP Chemistry Unit 3 Test Review Topics Covered: Gases Liquids
... Long Response: Calculator may be used. CLEARLY SHOW THE METHOD USED AND STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your advantage to do this, because you may earn partial credit if you do and you will receive little or no credit if you do not. Attention should be paid to significant figure ...
... Long Response: Calculator may be used. CLEARLY SHOW THE METHOD USED AND STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your advantage to do this, because you may earn partial credit if you do and you will receive little or no credit if you do not. Attention should be paid to significant figure ...
(1/V m C) +
... enhancement is greatest when the Plasmon frequency is in resonance with the radiation. In order for scattering to occur, the plasmon oscillations must be perpendicular to the surface. If they are in plane with the surface, no scattering will occur. It is because of this requirement that roughened su ...
... enhancement is greatest when the Plasmon frequency is in resonance with the radiation. In order for scattering to occur, the plasmon oscillations must be perpendicular to the surface. If they are in plane with the surface, no scattering will occur. It is because of this requirement that roughened su ...
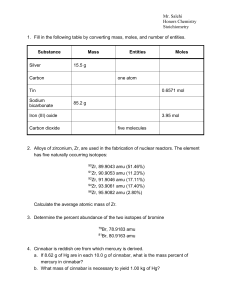
Stoichiometry - Cloudfront.net
... and produced 3.800 g of CO2 and 1.040 g of H2O. Assuming the compound contains only carbon, hydrogen, and oxygen, determine the empirical formula of the unknown compound. 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 ...
... and produced 3.800 g of CO2 and 1.040 g of H2O. Assuming the compound contains only carbon, hydrogen, and oxygen, determine the empirical formula of the unknown compound. 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 ...
Unit 8 Test Review
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
Thermochemistry Questions
... • When solutions containing silver ions and chloride ions are mixed, silver chloride precipitates: Ag+(aq)+Cl−(aq)→AgCl(s)ΔH=−65.5kJ a) Calculate ΔH for formation of 0.450mol of AgCl by this reaction. b) Calculate ΔH for the formation of 9.50g of AgCl. c) Calculate ΔH when 9.29×10−4mol of AgCl disso ...
... • When solutions containing silver ions and chloride ions are mixed, silver chloride precipitates: Ag+(aq)+Cl−(aq)→AgCl(s)ΔH=−65.5kJ a) Calculate ΔH for formation of 0.450mol of AgCl by this reaction. b) Calculate ΔH for the formation of 9.50g of AgCl. c) Calculate ΔH when 9.29×10−4mol of AgCl disso ...
AP CHEMISTRY – Source: 1999 AP Exam, Also Data Base of MC
... 26. According to the rate law for the reaction, an increase in the concentration of hydronium ion has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the equilibrium constant increases. (D) The value of the equilibrium constant ...
... 26. According to the rate law for the reaction, an increase in the concentration of hydronium ion has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the equilibrium constant increases. (D) The value of the equilibrium constant ...
Elaine Teto
... relative amounts of each substance present. However, in using these coefficients we come to our next topic, balancing chemical reactions. One of the most important laws to remember, when considering balancing equations is the Law of Conservation of Mass, which states that matter can neither be creat ...
... relative amounts of each substance present. However, in using these coefficients we come to our next topic, balancing chemical reactions. One of the most important laws to remember, when considering balancing equations is the Law of Conservation of Mass, which states that matter can neither be creat ...
Exam Review - hrsbstaff.ednet.ns.ca
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
... The reaction of solutions of ammonium phosphate and barium nitrate gives a precipitate of barium phosphate. The equation that best represents this statement is a) 2(NH4)3PO4(s) + 3Ba(NO3)2(aq) → Ba3(PO4)2(aq) + 6NH4NO3(s). b) 2(NH4)3PO4(aq) + 3Ba(NO3)2(aq) → Ba3(PO4)2(s) + 6NH4NO3(aq). c) 2(NH4)3PO4 ...
Ch 19 test_take-home
... D) the process is not spontaneous in either direction E) both forward and reverse processes have stopped 13) A reversible process is one that __________. A) can be reversed with no net change in either system or surroundings B) happens spontaneously C) is spontaneous in both directions D) must be ca ...
... D) the process is not spontaneous in either direction E) both forward and reverse processes have stopped 13) A reversible process is one that __________. A) can be reversed with no net change in either system or surroundings B) happens spontaneously C) is spontaneous in both directions D) must be ca ...